The overarching goal of the Arizona Twin Project addresses a central developmental question — how resilience (the capacity to bounce back following adversity) develops and affects the impact of early risk on child physical health and common mental health disorders, such as attention-deficit hyperactivity disorder, conduct problems, anxiety and depression. The study begins in infancy and longitudinally follows the twins across childhood, with plans to continue to follow them across adolescence into adulthood. There is an urgent need to understand these processes, as health problems continue to rise among children and adolescents, with adolescents spending less time sleeping, having higher body mass, reporting more depression, self-harm, conduct problems and peer problems (Keyes et al., Reference Keyes, Maslowsky, Hamilton and Schulenberg2015; Patalay & Gage, Reference Patalay and Gage2019). By focusing on components of early resilience within a representative twin study, our goal is to elucidate processes by which youth bounce back after adversity and grow to be healthy and competent individuals.
The main focus of the Arizona Twin Project involves understanding the genetic and environmental influences within and across multiple domains of development. The Arizona Twin Project spans individual, family, neighborhood and sociocultural levels of analysis, with an emphasis on sleep, pain, physiological stress processes, mental and physical health, and academic competence. Importantly, our ethnically diverse sample is representative of the state of Arizona, thus affording the opportunity to consider the role of culture in the etiology of various child outcomes. In sum, the Arizona Twin Project utilizes behavior genetic methods in order to understand risk and resilience processes that promote healthy development.
Recruitment and Retention
The Arizona Twin Project is an ongoing longitudinal study involving 350 pairs of young twins, 32% monozygotic (MZ) twins, 36% same-sex dizygotic (DZ), 32% opposite-sex DZ, currently 10–11 years of age. Twin participants are diverse in terms of race/ethnicity (>31% Latinx) and socioeconomic status (SES) (45.8% below middle class). Income ranges from less than US $20K to over $100K, with a median of $50–70k. Parental education ranges from less than a high school degree to a professional degree, with mean education of ‘some college’.
Families were recruited when twins were 10 months of age through collaboration with the Arizona State Department of Health Services, who sent recruitment letters (in English and Spanish) to mothers (>18 years) who had given birth to live twins in an Arizona hospital between July 2007 and July 2008. The letter included a postage-paid return letter where families could indicate their interest in participating, and follow-up letters were mailed 1 month after the first letter if we did not receive a response. Informed consent was obtained prior to all interviews, and participants were monetarily compensated at each measurement occasion.
We have regular contact with the families (i.e., sending our quarterly Arizona Twin Project Newsletter and birthday cards), which keeps contact information current, and we provide flexibility in scheduling assessments. Attrition from infancy to 2, 5, 8, 9 and 10 years has been <16%, with those who participated only at 1 year similar to other families on our key health variables.
The Zygosity Questionnaire for Young Twins (Goldsmith, Reference Goldsmith1991) was used to determine whether twins were identical or fraternal. This questionnaire yields over 95% agreement with zygosity determined via genotyping (Forget-Dubois et al., Reference Forget-Dubois, Perusse, Turecki, Girard, Billette, Rouleau and Tremblay2003; Price et al., Reference Price, Freeman, Craig, Petrill, Ebersole and Plomin2000). Zygosity was further verified with infant birth medical records and photographs taken at home visits. In addition, we genotyped the 3% most difficult cases.
Middle Childhood Data Collection: 8–11 Years of Age
The previous Arizona Twin Project overview describes findings from the prenatal, infancy and early childhood assessments (Lemery-Chalfant et al., Reference Lemery-Chalfant, Clifford, McDonald, O’Brien and Valiente2013). Table 1 lists completed assessments at 8–11 years. Demographic variables such as SES (including subjective and objective assessments and income-to-needs ratio), food insecurity, race/ethnicity and number of adults and children in the home are assessed at each occasion. All measures have been used in previous research and have acceptable levels of reliability and validity with White samples. Whenever possible, we used measures that have also been used with Latinx samples, and we statistically examine measurement equivalence across ethnic groups.
Table 1. Arizona Twin Project middle childhood assessments
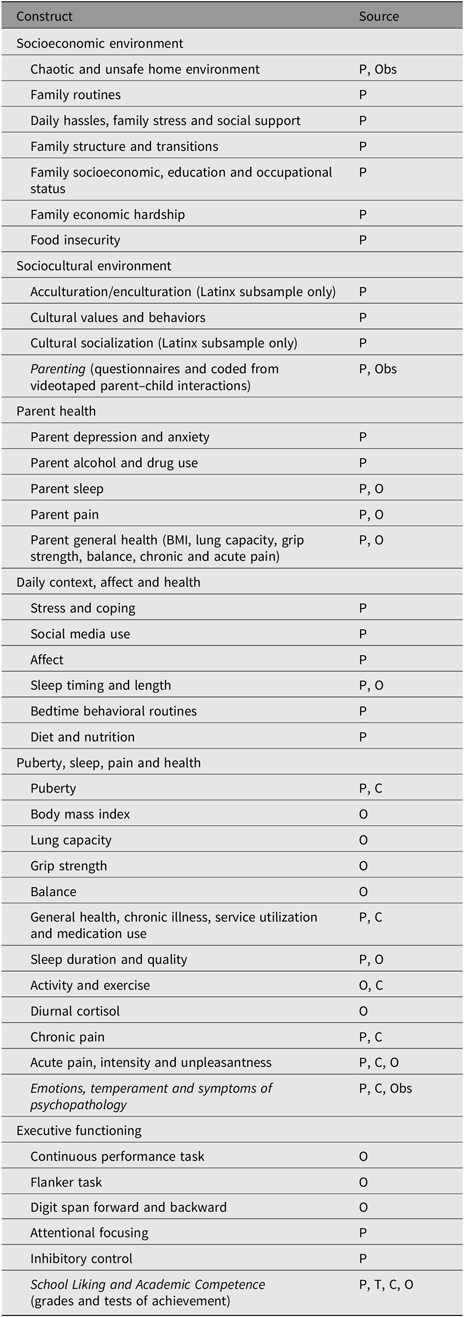
Note: 1 and 2.5 year assessments are described in Lemery-Chalfant et al. (Reference Lemery-Chalfant, Clifford, McDonald, O’Brien and Valiente2013). P = parent (mothers and fathers); T = Teacher; Obs = Observed; C = Child; O = Objective.
General Home Visit Protocol
After obtaining primary caregiver consent and youth assent, the primary caregiver and each twin (first one randomly selected, then the other) participate in a videotaped parent–child discussion task, and staff conduct the HOME assessment of cognitive, social-emotional and physical support within the home environment (Bradley et al., Reference Bradley, Corwyn, Caldwell, Whiteside-Mansell, Wasserman and Mink2000). Twins independently complete working memory and executive functioning tasks with staff (i.e., Digit Span; Wechsler, Reference Wechsler2003) and on laptop computers using the Psychology Experiment Building Language (Mueller, Reference Mueller2013). Tasks include the continuous performance task (Conners, Reference Conners2000) and the Eriksen flanker task (Eriksen & Eriksen, Reference Eriksen and Eriksen1974). The cold pressor task (Von Baeyer et al., Reference von Baeyer, Piira, Chambers, Trapanotto and Zeltzer2005) is used to assess acute pain tolerance, repeated with and without the primary caregiver present. Objective assessments of health are also obtained, including measures of lung capacity, grip strength, balance and BMI. Home visits last 2–3 h including breaks to minimize fatigue.
Actigraphy Assessment of Sleep and Activity
The Motionlogger Micro Watch, a wrist-based accelerometer, (Ambulatory Monitoring, Inc., Ardsley, NY, USA) is worn on the children’s nondominant wrist for 7 days and nights. These devices measure motion in 1-min epochs using a zero-crossing mode. Sleep measures extracted include (1) sleep midpoint time (halfway point between wake and bed time), (2) hours of sleep (sleep time excluding all periods of wakefulness during total sleep period), (3) sleep efficiency (time asleep divided by time in bed) and (4) sleep latency (minutes in bed before falling asleep). Indicators of variability are also constructed. Activity measures include (1) mean duration of wake periods, (2) mean activity level, (3) moderate or vigorous activity (reaching activity counts above age-specified cutoffs) and (4) sedentary activity.
Reports of Chronic Pain and Assessments of Acute Pain
Primary caregivers and twins both report on child recurring pain in the last 3 months by reporting on the frequency of pain in different body locations on a 1 (never/rarely) to 5 (about every day) scale. Additionally, caregivers complete survey assessments of clinical pain in five locations for each twin: headache, stomachache, lower back pain, widespread pain and temporomandibular pain (Dionne et al., Reference Dionne, Dunn, Croft, Nachemson, Buchbinder, Walker and Hartvigsen2008; Drossman, Reference Drossman2016; Dworkin et al., Reference Dworkin, Von Korff and LeResche1992; Headache Classification Subcommittee of the International Headache Society, 2004; Wolfe et al., Reference Wolfe, Clauw, Fitzcharles, Goldenberg, Katz, Mease and Yunus2010). Acute pain is assessed with the cold pressor task, during which children immerse their hand in ice cold water (50°± 1° Fahrenheit) and keep it underwater for as long as they can (up to 4 min). Each twin rates their pain after the task via the Faces of Pain Scale-Revised (Hicks et al., Reference Hicks, von Baeyer, Spafford, van Korlaar and Goodenough2001) and the Facial Affective Scale (McGrath et al., Reference McGrath, De Veber and Hearn1985). Twins complete this task twice and are randomized to complete their first trial either alone or with their primary caregiver.
Preliminary Findings
The previous Arizona Twin Project overview describes findings from the infancy and early childhood assessments (Lemery-Chalfant et al., Reference Lemery-Chalfant, Clifford, McDonald, O’Brien and Valiente2013). We have begun to analyze data from the middle childhood assessments (8–11 years) and highlight a few recent findings below.
Sleep and Weight
Associations between sleep duration and increased body weight have been consistently reported by both cross-sectional and longitudinal studies (Magee & Hale, Reference Magee and Hale2012), yet little is known regarding potential underlying mechanisms. We tested the extent to which associations between sleep and weight indicators were genetically or environmentally mediated. Results indicated that associations were explained by shared additive genetic factors (3%–17% of variance), suggesting that a common, underlying set of genes likely explains relations between sleep problems and high weight and adiposity in children (Breitenstein et al., Reference Breitenstein, Doane, Clifford, Miadich and Lemery-Chalfant2018). However, links between parent-reported and objective sleep indicators were primarily accounted for by shared environmental factors (5%–65% of variance), suggesting that subjective and objective measures may capture different traits (Breitenstein et al., Reference Breitenstein, Doane and Lemery-Chalfant2019). Self-regulatory effortful control was also linked with both sleep and weight indicators, such that links between effortful control and objective sleep (1%–3% of variance) and weight indicators (2% of variance) were explained by shared additive genetics, whereas the association between effortful control and parent-reported sleep was explained by shared environmental factors (5% of variance; Breitenstein, Reference Breitenstein2019).
Impulsivity and Sleep
Impulsivity has been associated with shorter sleep duration among children (Gruber et al., Reference Gruber, Cassoff, Frenette, Wiebe and Carrier2012); however, the underlying mechanisms are again unclear. We examined whether associations between impulsivity and objectively assessed sleep duration and efficiency were genetically or environmentally influenced. Results indicated common genetic influences and unique environmental contributions to impulsivity and sleep. Additive genetic correlations were .24 for associations with sleep duration and .23 for sleep efficiency (Miadich et al., Reference Miadich, Doane, Breitenstein, Clifford, Davis and Lemery-Chalfant2018).
Moderation of Genetic and Environmental Influences on Executive Functioning
A common factor of executive functioning has been found to be highly heritable in childhood (Engelhardt et al., Reference Engelhardt, Briley, Mann, Harden and Tucker-Drob2015), though little is known regarding variable etiologies of executive functioning across contexts. We examined whether early-life family stress and SES moderated the genetic and environmental influences on executive functioning at 8 years (Rea-Sandin et al., Reference Rea-Sandin, Clifford, Doane and Lemery-Chalfant2018). Measures of executive functioning included computerized continuous performance and flanker tasks, digit span forward and backward, and parent-reported attentional focusing and inhibitory control. The measures were included in a principal component analysis to index a common executive functioning factor. As family stress increased, the role of the shared environment increased for inhibitory control, Δχ 2(2) = 6.14, p = .05. The role of the shared environment also increased for digit span backward as SES increased, Δχ 2(2) = 1.86, p = .40. Although moderation was observed for select executive functioning variables, the etiology of the common executive functioning factor was not moderated by early family stress or SES, suggesting that etiology is constant across stressful childhood environments.
We also examined whether familism, an important cultural value characterized by valuing obedience and respect for family members, moderated the etiology of executive functioning in middle childhood (Rea-Sandin et al., Reference Rea-Sandin, Doane, Gonzales and Lemery-Chalfant2019). Although traditionally endorsed by Mexican American individuals, we found that other American ethnic groups ascribe to familism values as well. Results indicated that the nonshared environmental influence on common executive functioning increased as familism increased, Δχ 2(2) = 3.86, p = .15. Our findings highlight the need for genetically informed research to consider the role of culture on the genetic and environmental influences on children’s health.
Moderation of Genetic and Environmental Influences on Parenting
Traditional examinations of parenting as a causal factor influencing children’s development diminish the fact that children’s heritable traits affect parenting behaviors, representing an evocative gene–environment correlation (Avinun & Knafo, Reference Avinun and Knafo2014). We examined whether the degree of genetic and environmental influence on observed negative parenting practices varied as a function of SES and found that heritability was greatest at the extremes of SES and negligible at average levels, whereas variance attributable to shared environmental influence declined as SES increased, Δχ 2(1) = 1.54, p = .21. Total variance in negative parenting also decreased as SES increased (Oro et al., Reference Oro, Doane and Lemery-Chalfant2019). Results suggest that factors operating at both the low and high extremes of SES may contribute to parents being more reactive to their children’s heritable traits.
Children’s Chronic Pain, Acute Pain and Internalizing Symptoms
There is a robust area of research examining both the etiology of and relations among chronic pain, acute pain and mood disorder symptoms in adulthood; however, parallel research in childhood is quite limited. With a partial sample, we examined the extent to which children’s chronic pain (primary caregiver report), acute pain (duration of cold pressor task) and internalizing symptoms (self-report) were genetically and environmentally influenced as well as phenotypically related. Results yielded moderate to high estimates of heritability, with 43%, 73% and 34% of the variance in chronic pain, acute pain and internalizing symptoms explained by genetic factors, respectively. Phenotypic correlations indicated that children’s chronic pain, acute pain and internalizing symptoms were relatively independent, a departure from the adult literature warranting further examination from a developmental perspective (preliminary analysis, 2019).
Pain Transmission within Families
Several studies report evidence for the familial aggregation of pain symptoms, but these studies overwhelmingly focus on adult relatives and offspring. We examined associations between primary caregivers’ and children’s pain and internalizing symptoms. Results indicated significant within domain correlations, but not across domains. Intergenerational correlations for chronic pain, acute pain and internalizing were .21 (p < .001), .22 (p < .001) and .25 (p < .001), respectively (preliminary analysis, 2019). These findings suggest that pain transmission within families occurs as early as middle childhood.
These preliminary findings attest to the critical influence of the sociocultural and socioeconomic contexts on health in middle childhood, both directly and as a moderator of genetic and environmental etiology. However, data collection is ongoing so important longitudinal models have yet to be tested. Future analyses will elucidate developmental cascades capturing dynamic progressions across the mental and physical health domains.
Future Directions
Currently, we are seeking funding for assessments in adolescence, focused on how the dynamic developmental processes of puberty, sleep and general health assessed at 8–12 years predict inflammation and mental health at age 14 years. Our extensive environmental assessment includes Geographic Information Systems coding of neighborhoods, as well as daily assessment of frequency and duration of media use.
Conclusion
The longitudinal Arizona Twin Project has been ongoing since 2007, with annual in-depth assessments across middle childhood funded by the US National Institutes of Health (R01HD079520 and R01HD086085). Twins were recruited through birth records, with significant representation of Latinx, as well as White participants. Multiple aspects of the prenatal, infancy and childhood physical and sociocultural environments have been assessed, creating an opportunity to study stability and change, gene–environment correlation and moderated heritability. In-depth phenotyping of physical and mental health outcomes allows comparison across methods. We welcome collaboration with other twin researchers and panels. Results contribute to a growing area of research on the intersection of cultural and genetic approaches to the study of physical and mental health.
Acknowledgments
Support for this research was provided by the US Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01HD079520 and R01HD086085). We also wish to extend our appreciation to the Arizona Twin Project students and staff, and the participating twins and families.



