Adherence to antipsychotic medication has been shown to be the single most important determinant of relapse in schizophrenia. Reference Robinson, Woerner, Alvir, Bilder, Goldman and Geisler1 Compared with oral antipsychotics, long-acting injections are associated with better global outcome, reduced risk of hospitalisation and longer times to discontinuation. Reference Carpenter, Hanlon, Heinrichs, Summerfelt, Kirkpatrick and Levine2,Reference Herz, Glazer, Mostert, Sheard, Szymanski and Hafez3 Risperidone long-acting injection is the first of the second-generation antipsychotics to be available in depot or long-acting formulation and has been used in routine UK clinical practice since 2002. Reference Kane, Eerdekens, Lindenmayer, Keith, Lesem and Karcher4 There is little research to inform prescribing decisions in the clinic between the various long-acting injections. Meta-analytic review of first-generation depots found little difference between individual medications. Reference Adams, Fenton, Quraishi and David5 No direct comparisons of risperidone long-acting injection with the first-generation depots are available except for one open, 6-month randomised study that showed favourable outcome for risperidone long-acting injection compared with zuclopenthixol decanoate for individuals with comorbid substance misuse. Reference Rubio, Martinez, Ponce, Jimenez-Arriero, Lopez-Munoz and Alamo6 Due to the growing trend towards the use of second-generation antipsychotics in general, 7 including risperidone long-acting injection, despite the lack of head-to-head evidence noted above, we aimed to retrospectively identify and measure the outcome of patients started on: risperidone long-acting injection, zuclopenthixol decanoate, flupentixol decanoate, fluphenazine decanoate, pipothiazine palmitate and haloperidol decanoate. To assess effectiveness we applied the Clinical Global Impression (CGI) scale Reference Guy8 and measured discontinuation rates and time to hospitalisation after the long-acting injection was started.
Method
The electronic patient records covering all secondary care contacts for psychiatry in a discrete geographic area (the county of Lanarkshire, Scotland, population 550 000) were examined. The electronic records were phased into NHS Lanarkshire's mental health service over the period 2002-2005 (Motherwell/Clydesdale district in 2002, Hairmyres/East Kilbride in 2004 and Monklands District in 2005) into general, rehabilitation, liaison, addiction and forensic psychiatry services. Therefore, some of the ‘oldest’ records ran from February 2002 until October 2008. There are no private or independent secondary psychiatric services in Lanarkshire, and no intensive home-based alternatives to hospitalisation exist. All individuals in mental healthcare follow-up have a patient record. A total of approximately 35 000 individual records were available and were searched for the keywords relating to the generic and UK trade names of all the aforementioned depot antipsychotic injections. The ICD-10 9 diagnoses included in our study were schizophrenia (F20), persistent delusional disorders (F22) and schizoaffective disorders (F25). All other ICD-10 diagnoses were excluded. Patient records resulting from this search that were considered inadequate for analysis (i.e. those where the drug was started before the electronic record became available or those with only a single mental health contact) were excluded. No other exclusion criteria were applied.
Demographic and clinical variables
These were extracted from the records and the results are shown in online Table DS1. Additional concurrent antipsychotics were defined as being another regular (not ‘as required’) antipsychotic drug prescribed at least 50% of the time that individuals were on the depots. This was quantified by converting doses to percentage of British National Formulary (BNF) defined maximum dosage. 10 For example, 100 mg per day of chlorpromazine is 10% of the maximum BNF daily dose. This measure is important in our clinical practice where BNF-defined maximum dosages are linked to high-dose antipsychotic protocols.
Clinical Global Impression
The clinical status of individuals was assessed using the Clinical Global Impression severity (CGI-S) and improvement (CGI-I) scales. The proportion who improved as defined by CGI-I scores 1-4 (very much improved through to minimally improved) was the primary outcome measure. The rationale for this broad definition was that in clinical practice any degree of improvement is of potential value as opposed to clinical trials where more stringent criteria tend to be employed. Our CGI scores were based on records and assigned retrospectively by experienced psychiatrists (E.S., D.D. and P.S.), all having a minimum 7 years postgraduate experience in psychiatry. Our interrater reliability studies for these measures resulted in high levels of agreement (kappa >0.8 for 60 records examined by three raters). Severity rating was assigned at the start of treatment, at approximately 3-5 months after onset of treatment and at the end of treatment if the drug was discontinued or at the end of the medical record. The reason for examining severity at 3-5 months post depot initiation was that there were anecdotal reports of risperidone long-acting injection taking longer to show clinical benefit compared with other depots. Improvement scores were assigned as a result of the perceived effects of the medication and therefore took into account baseline severity of illness. Such retrospective CGI assignment has been used previously for examining clinical response to antipsychotics. Reference Barbee, Conrad and Jamhour11-Reference Shajahan, MacRae, Bashir and Taylor13
Discontinuation and hospitalisation
Time to treatment discontinuation is increasingly used as a primary outcome measure in antipsychotic effectiveness research. Reference Stroup, Lieberman, McEvoy, Swartz, Davis and Capuano14-Reference Jones, Barnes, Davies, Dunn, Lloyd and Hayhurst16 Time to discontinuation was examined for any causes and subcategorised into time to discontinuation as a result of inefficacy or adverse effects. When more than one reason for discontinuation was noted, we used the clinically most important reason identified after reviewing the record for the statistical analyses. Time to admission to hospital (mental health admission unit) was recorded as a further measure of effectiveness and is also considered a putative marker of antipsychotic treatment failure. Reference Kahn, Fleischhacker, Boter, Davidson, Vergouwe and Keet17,Reference Rabinowitz, Lichtenberg, Kaplan, Mark, Nahon and Davidson18
Statistical analysis
StatsDirect (www.statsdirect.com) was used on Windows XP to perform our statistical analyses. Continuous data were reported as means with 95% CI and compared using analysis of variance and t-tests. Categorical and non-parametric data were analysed using χ2-tests and log-transformed as appropriate. Significance levels required two-tailed P<0.05. Kaplan-Meier survival curves were used to illustrate the probability of treatment discontinuation or hospitalisation over time. Hazard ratios (HRs) were calculated for survival analyses, and survival curves were compared using non-parametric methods with no assumptions about the distributions of survival estimates. Our previous studies on oral antipsychotic effectiveness showed that prior or subsequent treatment with clozapine (a putative marker of treatment resistance) and affective symptoms had an effect on proportional CGI improvement (less improvement with clozapine, more with schizoaffective disorder). Reference Shajahan, MacRae, Bashir and Taylor13,Reference Rabinowitz, Lichtenberg, Kaplan, Mark, Nahon and Davidson18 For these reasons, analyses were performed on all participants and separately after excluding individuals with treatment resistance and schizoaffective disorder.
Results
A total of 811 individuals were identified as having records mentioning that they had ever been on the depots being studied. Of these 811 people, 259 had been started on depots after the electronic document management system had become available. Of these, 84% (n = 217) had a diagnosis of schizophrenia, schizoaffective disorder or related psychosis. The proportion of people started on risperidone long-acting injection exceeded the cumulative total of those started on the other depots, illustrating its prescriber preference over first-generation depot antipsychotics during 2002-2008. Numerically, people started on fluphenazine decanoate, pipothiazine palmitate and haloperidol decanoate were small. For comparison purposes they were included in the study, although statistical analysis was restricted to risperidone long-acting injection, zuclopenthixol decanoate and flupentixol decanoate.
Online Table DS1 shows the inclusive nature of the study population. A significant proportion of the whole sample were women (38%), 24% were undergoing compulsory treatment, 29-47% had a lifetime history of alcohol or substance misuse, 9-42% were taking antidepressant and mood-stabilising medications, there was 30% antipsychotic polypharmacy and 23% treatment resistance (as defined by prior or subsequent clozapine use or consideration as recorded by clinicians in case records). The majority of individuals (89%) were switching immediately from another antipsychotic. Those remaining were usually being recommenced on a depot after a period of non-adherence with treatment. There was a trend for zuclopenthixol being started when the previous antipsychotic was discontinued owing to inefficacy. There was a mean period of 15.6-18.5 months before the three main depot antipsychotics (risperidone long-acting injection, zuclopenthixol decanoate and flupentixol decanoate) were introduced. This allowed for extraction of useful clinical information prior to the depot being started. A total of 25 different consultant psychiatrists were involved in the initiation of the three main depots studied (25 consultants for risperidone long-acting injections (n = 122), 11 consultants for zuclopenthixol decanoate (n = 31) and 19 consultants for flupentixol decanoate (n = 43). More records originated from one particular district than from others (because of the phased introduction of records); however, there was no statistically significant difference in the type of long-acting injection according to district. There were differences in the mean total duration of records between the three main long-acting injections studied, with zuclopenthixol decanoate records being about 8 months shorter than risperidone long-acting injection or flupentixol decanoate records (P = 0.054). However, the mean duration of treatment did not differ significantly between the long-acting injections. In total, the study incorporated 283 patient-years of new start depot antipsychotic experience from 2002 to 2008.
Table 1 shows CGI-S and GCI-I scores. Adjusted results after excluding individuals with schizoaffective disorder and those who were treatment resistant showed similar patterns, with statistical significance remaining. Flupentixol decanoate was started for people with a lower severity of illness score compared with zuclopenthixol decanoate (P = 0.003) or risperidone long-acting injection (P = 0.018). After 3-5 months, CGI-S scores were lower with flupentixol compared with risperidone long-acting injection (P = 0.038). Between 72 and 74% of individuals made at least some degree of clinical improvement following the commencement of risperidone long-acting injection, zuclopenthixol decanoate or flupentixol decanoate. Within the CGI-I categories (1-8), fewer people had ‘very much improved’ or ‘much improved’ (CGI-I scores of 1 or 2) after commencing zuclopenthixol decanoate compared with risperidone long-acting injection or flupentixol decanoate.
Table 1 Clinical Global Impression scores and duration of treatment

| Risperidone long-acting injection (n = 122) | Zuclopenthixol decanoate (n = 31) | Flupenthixol decanoate (n = 43) | Fluphenazine decanoate (n = 11) | Pipothiazine palmitate (n = 7) | Haloperidol decanoate (n = 3) | P a | |
|---|---|---|---|---|---|---|---|
| CGI–S at onset of treatment, mean score (95% CI) | 4.5 (4.3–4.7) | 4.8 (4.5–5.0) | 4.1 (3.8–4.4) | 4.9 (4.5–5.3) | 4.4 (3.9–4.9) | 4.7 (3.2–6.1) | 0.0026a |
| CGI–S at 3–5 months after starting depot, mean score (95% CI) | 3.3 (3.0–3.5) | 3.1 (2.9–3.4) | 2.6 (2.1–3.1) | 4.6 (3.5–5.7) | 3.0 (1.7–4.3) | 0.038b | |
| CGI–S improvement after 3–5 months of treatment, mean % (95% CI) | 24.7 (19.7–29.8) | 32.6 (25.6–39.5) | 36.4 (26.6–46.3) | NS | |||
| CGI–S at end of treatment or record, mean score (95% CI) | 3.3 (3.1–3.5) | 3.1 (2.8–3.4) | 2.9 (2.6–3.3) | 4.1 (3.5–4.6) | 3.1 (2.0–4.3) | 2.7 (1.1–6.5) | NS |
| CGI–S improvement from onset of treatment, mean % (95% CI) | 24.7 (19.7–29.8) | 33.0 (25.3–40.6) | 27.8 (19.9–35.8) | NS | |||
| CGI–I, mean score (95% CI) | 3.5 (3.2–3.7) | 3.4 (3.0–3.8) | 3.3 (2.9–3.7) | 4.2 (3.7–4.9) | 3.1 (1.9–4.4) | 2.0 (0.5–4.5) | NS |
| Improved (CGI–I <5) all patients, n (%) | 90 (74) | 23 (74) | 31 (72) | 5 (45) | 6 (86) | 3 (100) | NS |
| CGI–I, n (%) | |||||||
| 1 Very much improved | 13 (10.7) | 0 (0) | 3 (7.0) | 0 (0) | 1 (14.3) | 1 (33.3) | 0.0007c |
| 2 Much improved | 22 (18.0) | 5 (16.1) | 13 (30.2) | 0 (0) | 1 (14.3) | 1 (33.3) | |
| 3 Moderately improved | 26 (21.3) | 17 (54.8) | 9 (20.9) | 3 (27.3) | 2 (28.6) | 1 (33.3) | |
| 4 Minimally improved | 29 (23.8) | 1 (3.2) | 6 (14.0) | 2 (18.2) | 2 (28.6) | 0 (0) | |
| 5 No change | 24 (19.6) | 8 (25.8) | 10 (23.3) | 6 (54.5) | 1 (14.3) | 0 (0) | |
| 6 Minimally worse | 5 (4.1) | 0 (0) | 2 (4.7) | 0 (0) | 0 (0) | 0 (0) | |
| 7 Moderately worse | 3 (2.4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| 8 Much worse | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Duration of treatment with depot, months: mean (95% CI) | 16.4 (13.9–18.9) | 17.1 (12.5–21.7) | 13.6 (9.8–17.5) | 7.3 (1.1–13.5) | 18.1 (1.1–35.2) | 27.9 (12.8–68.6) | NS |
Figure 1 depicts Kaplan-Meier survival curves illustrating time to discontinuation for any cause, inefficacy, side-effects and time to hospital admission. For all causes and specifically as a result of adverse effects and inefficacy, zuclopenthixol decanoate was less likely to be discontinued compared with risperidone long-acting injection or flupentixol decanoate. Survival curves after excluding those individuals who were treatment resistant or had schizoaffective disorder showed similar results. The statistics for these survival curves are presented in Table 2.

Fig 1 Kaplan-Meier survival curves (unadjusted) - time to treatment discontinuation because of (a) all causes, (b) inefficacy, (c) side-effects and (d) time to hospitalisation. RLAI, risperidone long-acting injection.
Table 2 Treatment discontinuation and hospitalisation hazard ratios (HRs)
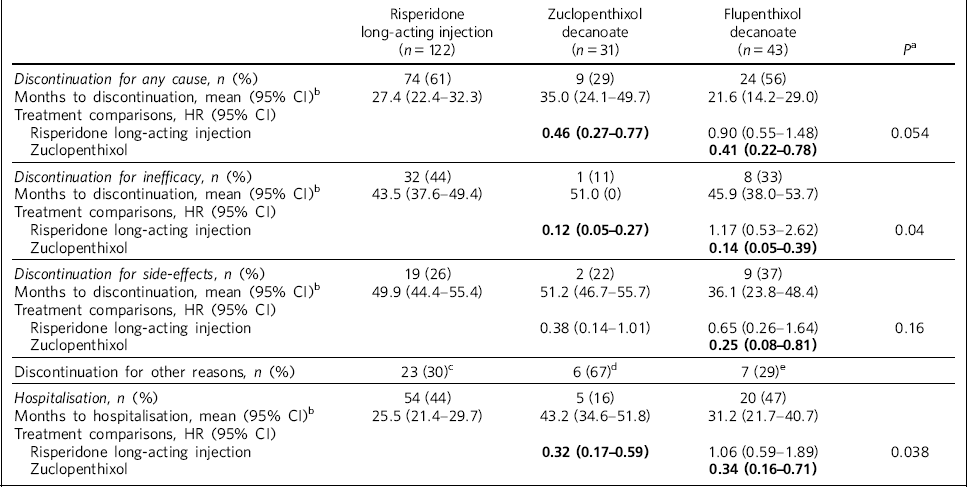
| Risperidone long-acting injection (n = 122) | Zuclopenthixol decanoate (n = 31) | Flupenthixol decanoate (n = 43) | P a | |
|---|---|---|---|---|
| Discontinuation for any cause, n (%) | 74 (61) | 9 (29) | 24 (56) | |
| Months to discontinuation, mean (95% CI)b | 27.4 (22.4–32.3) | 35.0 (24.1–49.7) | 21.6 (14.2–29.0) | |
| Treatment comparisons, HR (95% CI) | ||||
| Risperidone long-acting injection | 0.46 (0.27–0.77) | 0.90 (0.55–1.48) | 0.054 | |
| Zuclopenthixol | 0.41 (0.22–0.78) | |||
| Discontinuation for inefficacy, n (%) | 32 (44) | 1 (11) | 8 (33) | |
| Months to discontinuation, mean (95% CI)b | 43.5 (37.6–49.4) | 51.0 (0) | 45.9 (38.0–53.7) | |
| Treatment comparisons, HR (95% CI) | ||||
| Risperidone long-acting injection | 0.12 (0.05–0.27) | 1.17 (0.53–2.62) | 0.04 | |
| Zuclopenthixol | 0.14 (0.05–0.39) | |||
| Discontinuation for side-effects, n (%) | 19 (26) | 2 (22) | 9 (37) | |
| Months to discontinuation, mean (95% CI)b | 49.9 (44.4–55.4) | 51.2 (46.7–55.7) | 36.1 (23.8–48.4) | |
| Treatment comparisons, HR (95% CI) | ||||
| Risperidone long-acting injection | 0.38 (0.14–1.01) | 0.65 (0.26–1.64) | 0.16 | |
| Zuclopenthixol | 0.25 (0.08–0.81) | |||
| Discontinuation for other reasons, n (%) | 23 (30)c | 6 (67)d | 7 (29)e | |
| Hospitalisation, n (%) | 54 (44) | 5 (16) | 20 (47) | |
| Months to hospitalisation, mean (95% CI)b | 25.5 (21.4–29.7) | 43.2 (34.6–51.8) | 31.2 (21.7–40.7) | |
| Treatment comparisons, HR (95% CI) | ||||
| Risperidone long-acting injection | 0.32 (0.17–0.59) | 1.06 (0.59–1.89) | 0.038 | |
| Zuclopenthixol | 0.34 (0.16–0.71) |
Any-cause discontinuation differed significantly between zuclopenthixol decanoate and risperidone long-acting injection (HR = 0.46, 95% CI 0.27-0.77) and flupentixol decanoate (HR = 0.41, 95% CI 0.22-0.78). Discontinuation as a result of inefficacy differed between zuclopenthixol decanoate and risperidone long-acting injection (HR = 0.12, 95% CI 0.05-0.27) and flupentixol decanoate (HR = 0.14, 95% CI 0.05-0.39). The likelihood of hospitalisation differed between zuclopenthixol decanoate and risperidone long-acting injection (HR = 0.32, 95% CI 0.17-0.59) and flupentixol decanoate (HR = 0.34, 95% CI 0.16-0.71).
Discussion
Principal findings
Over the period 2002-2008, we observed a trend towards increasing use of long-acting risperidone over first-generation long-acting antipsychotic injections. This is consistent with the trend seen in the increasing use of newer antipsychotics in other parts of the UK. 7 Most (76%) of the sample on long-acting injections were not detained and hence were receiving the injections on a voluntary basis. Co-prescription of antidepressants occurred in up to 51% of this group of people with chronic schizophrenia and additional oral antipsychotics were required in up to 40%. In terms of percentage of individuals showing any degree of CGI improvement, there was no difference between the three main depots (72-74% improved) although there were fewer people in the ‘very much improved’ or ‘much improved’ groups with zuclopenthixol decanoate compared with risperidone long-acting injection and flupentixol decanoate. Those started on risperidone long-acting injection who achieved ‘very much improved’ on the CGI had a higher initial illness severity to start with (two-tailed t-test P<0.001) and were less likely to have been tried on clozapine. Time to discontinuation as a result of inefficacy and time to hospitalisation favoured zuclopenthixol decanoate over risperidone long-acting injection and flupentixol decanoate. Time to discontinuation as a result of side-effects did not differ between the three depots. Second-generation antipsychotics were marketed on their superior side-effect profile and although we were unable to examine side-effects during treatment, discontinuation because of side-effects did not differ significantly with risperidone long-acting injection compared with zuclopenthixol decanoate or flupentixol decanoate.
Methodological issues
All typed correspondence from clinicians was uploaded into the electronic document management system in NHS Lanarkshire mental health services and the record is considered an effective duplication of the correspondence section of paper-based case records. As individuals on depot medication are usually within secondary care services and have repeated, usually multidisciplinary contacts, we are confident that our electronic records, which include medical, nursing and occupational therapy documents, captured a comprehensive and accurate picture of clinical contacts for all patients in Lanarkshire on the depot antipsychotics studied.
The possibility that the phased introduction of the electronic records, the recommendations of a minority of psychiatrists or the different lengths of the electronic records may be responsible for the results seen also requires consideration. Records for zuclopenthixol decanoate were shorter in duration than for risperidone long-acting injection or flupentixol by approximately 8 months on average. Theoretically, this allows less time for discontinuation events; however, using Kaplan-Meier derived survival curves and mean times to discontinuation or hospitalisation takes this into account. In addition, the mean duration of treatment was similar for the three main depots studied. Therefore, it is unlikely that different duration of records explains the different discontinuation rates seen.
We considered that the lower discontinuation rates for zuclopenthixol decanoate may have reflected its use in more treatment-resistant individuals, similar to the situation with clozapine where clinicians feel they are limited by subsequent choices after treatment failure and are reluctant to discontinue. There was some evidence to support this in that more individuals were started on zuclopenthixol decanoate as a result of inefficacy (61%) compared with risperidone long-acting injection (39%) or flupentixol decanoate (49%), although this just failed to achieve statistical significance. Similarly, a greater proportion of people started on zuclopenthixol decanoate were treated compulsorily, although again, this was not statistically significant. However, there was evidence to refute that zuclopenthixol decanoate was reserved for more treatment-resistant individuals in that these patients were less likely to have been tried on clozapine and there was no difference in the duration of contact with psychiatric services. Overall, our data do not support that zuclopenthixol was being used as a ‘last resort’ medication that clinicians were reluctant to discontinue. Our knowledge of local clinical practice would also support this viewpoint.
Strengths and weakness
The electronic record system allowed us to study all patients who were started on the most commonly prescribed depot antipsychotics in secondary care mental health services in a discrete geographical region within a defined period. This meant we were able to include individuals with co-prescription of other psychotropic agents, with comorbid conditions such as alcohol and substance misuse, and those who would be unable to consent to clinical trials (e.g. high illness severity and detained patients), all of whom present frequently in clinical practice. Such inclusiveness also allows follow-up of individuals over a relatively long period (in some of our cases over 5 years) thereby offering outcome information beyond the acute illness phase. Therefore, this study maximises the generalisability of findings to everyday clinical practice, in keeping with the views of Adams et al that study populations need to be as representative and long term as possible. Reference Adams, Fenton, Quraishi and David5 The downside of our inclusiveness is that the ‘noise’ generated by many confounding variables (which would lead to exclusion from some clinical trials) may mask the efficacy signal from one particular compound. The selection of patients was not from strict a priori criteria but a reflection of clinician and patient choice in the decision to start a depot during a particular psychotic illness episode. The study population is predominantly White and middle-aged and so may not be necessarily generalisable to other specific populations, for example, young adults with first-episode psychosis.
The effectiveness measure employed (proportion improved according to CGI) is a clinically relevant one, reflecting everyday clinical review of patients and their response to treatment. The CGI scale was originally designed to be used prospectively and is undoubtedly a less sophisticated instrument than specific symptom rating scales, but has been used by ourselves Reference Shajahan, MacRae, Bashir and Taylor13,Reference Tiihonen, Walhbeck, Lonnqvist, Klaukka, Ioannidis and Volavka19 and others Reference Barbee, Conrad and Jamhour11,Reference Centorrino, Fogarty, Cimbolli, Salvatore, Thompson and Sani12 to identify clinical response retrospectively.
Time to discontinuation is increasingly used as a primary outcome measure in antipsychotic effectiveness research. Reference Stroup, Lieberman, McEvoy, Swartz, Davis and Capuano14-Reference Jones, Barnes, Davies, Dunn, Lloyd and Hayhurst16 It is a relatively unbiased measure and usually, although not always, signals treatment failure because of inefficacy, adverse effects, non-adherence or combinations of these. Both time to and rate of hospitalisation Reference Kahn, Fleischhacker, Boter, Davidson, Vergouwe and Keet17,Reference Rabinowitz, Lichtenberg, Kaplan, Mark, Nahon and Davidson18 may be considered as markers of treatment failure. However, from clinical practice, we know that the reasons for hospitalisation are varied and usually include risks of self-harm, risk of harm to others and adverse social circumstances. In our locality, non-hospital options (e.g. home treatment teams) were not available during the study period.
Implications for clinical practice
When considering our outcome measures that were less subject to potential bias (i.e. time to discontinuation and hospitalisation) zuclopenthixol decanoate was superior to risperidone long-acting injection and flupentixol. These findings are consistent with the meta-analytic review by Adams et al Reference Adams, Fenton, Quraishi and David5 that showed an advantage for zuclopenthixol decanoate over other first-generation depots in terms of discontinuation. However, when considering the CGI, which was arguably more prone to potential bias, zuclopenthixol was associated with fewer individuals in the ‘very much improved’ and ‘much improved’ categories compared with risperidone long-acting injection and flupentixol. Of interest was the use of zuclopenthixol decanoate in people with probably greater illness severity, suggesting clinician preference in its use when individuals were more severely unwell.






eLetters
No eLetters have been published for this article.