Although psychiatrists commonly acknowledge the considerable threat to public health posed by depression, the extent to which depression contributes to mortality and illness burden through interactions with physical illnesses is often underestimated. Taking the example of coronary heart disease, the most common cause of death in the UK, depression increases the risk of incident coronary heart disease 1.6 times, even after controlling for other cardiovascular risks (Reference RuguliesRugulies, 2003; Reference Wulsin and SingalWulsin & Singal, 2003). Furthermore, depression doubles the risk of death in those with established heart disease, such as following myocardial infarction (Reference Barth, Schumacher and Herrmann-LingenBarth et al, 2004; Reference van Melle, de Jonge and Spijkermanvan Melle et al, 2004). As survival after myocardial infarction has increased, attention has also focused on the adverse effects of depression on health-related quality of life (HRQoL) (Reference Rumsfeld and HoRumsfeld & Ho, 2005). Relevant studies, however, have only partially controlled for the influence of potential confounding factors (Reference Brown, Melville and GrayBrown et al, 1999; Reference Failde and RamosFailde & Ramos, 2000; Reference Pocock, Henderson and ClaytonPocock et al, 2000; Reference Taira, Seto and HoTaira et al, 2000; Reference McBurney, Eagle and Kline-RogersMcBurney et al, 2002; Reference van Jaarsveld, Sanderman and Ranchorvan Jaarsveld et al, 2002; Reference Izawa, Hirano and YamadaIzawa et al, 2004; Reference Kim, Henderson and PocokKim et al, 2005).
METHOD
We performed a prospective cohort study to investigate the extent to which depression and anxiety contributed to impairment in HRQoL 12 months after the patient had been admitted with their first myocardial infarction. We aimed to understand more fully among people with coronary heart disease the burden of illness that is attributable to depression. We controlled for socio-demographic and clinical characteristics at baseline, including comorbid, non-cardiac illnesses and any further cardiac events during the follow-up period. Since depression may develop in the months following myocardial infarction (Reference Blumenthal, Lett and BabyakBlumenthal et al, 2003; Reference Poston, Haddock and ConardPoston et al, 2003; Reference Dickens, Percival and McGowanDickens et al, 2004a ), we included depression severity measured at baseline and at 6-month follow-up as predictive variables. We tested the hypothesis that anxiety and depression would predict impairment in HRQoL 12 months following first myocardial infarction, after controlling for possible confounding factors.
The study was approved by the relevant local research ethics committees.
Sample
Consecutive patients admitted to four inner-city hospitals with suspected myocardial infarction were screened for this study, on average 3.6 (s.d.=3.3) days after admission. Individuals were recruited if they had no history of previous myocardial infarction, were aged less than 80 years and met World Health Organization criteria for myocardial infarction (Reference GutzwillerGutzwiller, 1994). Two of the following were required: history of typical chest pain, characteristic echocardiogram changes, a serial rise in cardiac enzyme creatine phosphokinase (CPK). People with severe mental illness (including severe depression with suicidal ideas) were excluded from the study. Written, informed consent was provided by participants after the procedures involved in the study had been fully explained.
Participants received treatment as usual, as determined by the attending hospital physician and primary care doctors. As this was an observational study, participants did not receive any additional therapy (e.g. antidepressants) based on their research assessments.
Baseline assessments
We recorded age, gender (male=1, female=2), ethnicity (White=1, Black and minority ethnic=0), marital status (single: yes=1, no=0), years of education, smoking history (past or current smoker=1, never smoked=0), past psychiatric history (yes=1, 1, no=0) and comorbid illnesses for which participants were receiving regular treatment (for each illness yes=1, no=0). Socioeconomic status, based on current or latest employment, was categorised into high or low according to the criteria of Goldthorpe and Hope (Reference Goldthorpe and HopeGoldthorpe & Hope, 1974), (lower status=1, higher status=2). Serum cholesterol was measured during admission.
Details of the myocardial infarction were recorded from the notes, with discussion with the attending cardiologist if clarification was required. We recorded site of infarction as determined by echocardiogram, categorised as anterior (including anterior, anterolateral, anteroseptal and apical myocardial infarctions, scored as 1) or other (scored as 2). Details of treatment, including reperfusion therapy, surgical intervention during index admission (angioplasty or coronary artery bypass graft) and discharge medication were also noted. We used three measures of severity of myocardial infarction. The Killip class, a 4-point scale, rated left ventricular function based on the presence of pulmonary rales, S3 gallop rhythm and peripheral hypoperfusion, with high scores representing worse function (Reference Killip and KimballKillip & Kimball, 1967). Creatine phosphokinase levels were measured on admission and on 2 subsequent days as an indication of the extent of myocardial damage. Finally, left ventricular ejection fraction measures (using echocardiogram) were recorded for 217 patients.
Anxiety and depression were assessed using the self-rated, 14-item Hospital Anxiety and Depression Scale (HADS), which scores the severity of the symptoms of anxiety (scored 0 to 21) and depression (scored 0 to 21). This measure was developed specifically for medically ill populations, and excludes bodily symptoms such as sleep disturbance, fatigue and pain that may be owing to physical illness (Reference Zigmond and SnaithZigmond & Snaith, 1983). The HADS has been widely used in people with medical illnesses, including myocardial infarction, and its factor structure has been confirmed (Reference HerrmannHerrmann, 1997; Reference Herrmann, Brand and KaminskyHerrmann et al, 1998; Reference Mayou, Gill and ThompsonMayou et al, 2000). At baseline, participants were instructed to complete the questionnaire to reflect their mood during the week before myocardial infarction, in order to avoid recording mood changes that occur as immediate reactions to the infarction and during time spent on the coronary care unit. In addition to using HADS subscores to measure severity of depression and anxiety, a HADS total score of 17 or above was taken to indicate probable major depressive disorder, as we have validated this score against a standardised research interview (Reference Dickens, McGowan and PercivalDickens et al, 2004b ). We did not aim to measure distress in the period immediately after infarction, and we have recorded the fact that HADS scores rated for the days following admission were significantly higher than those rated for the week before, which were used in this report (Reference Dickens, McGowan and PercivalDickens et al, 2004b ).
Participants completed the Illness Perceptions Questionnaire (Reference Weinman, Petrie and Moss–MorrisWeinman et al, 1996), which assesses patients’ perception of their illness in terms of:
-
(a) its bodily symptoms (‘identity’ scale – scored 0 to 14, with high scores indicating more reported symptoms);
-
(b) its likely duration (‘time-line’ – scored 1 to 5, with low score reflecting longer anticipated duration);
-
(c) the perceived consequences (scored 1 to 6, with low score indicating more serious perceived consequences);
-
(d) the likelihood of the illness being cured or controlled (scored 11 to 5, with to 5, with low score indicating more perceived control).
This scale has been used previously for people who have experienced myocardial infarction (Petrie et al, Reference Petrie, Weinman and Sharpe1996, Reference Petrie, Cameron and Ellis2002).
We used the self-reported Medical Outcomes Study SF–36 (Reference Ware and SherbourneWare & Sherbourne, 1992) to assess both the physical and mental aspects of HRQoL at baseline, 6 months and 12 months following myocardial infarction. The SF–36 is a generic measure of HRQoL that provides scores on eight sub-scales, reflecting functioning over the month preceding the assessment. The SF–36 has been well validated in populations with coronary heart disease (Reference Failde and RamosFailde & Ramos, 2000; Reference Morrin, Black and ReidMorrin et al, 2000; Reference Rumsfeld and HoRumsfeld & Ho, 2005). We deliberately chose a generic measure of HRQoL, as opposed to a cardiac-specific measure, as our main outcome; people after myocardial infarction frequently experience multiple health problems, and we wanted a measure that was sensitive to the impact of noncardiac illnesses on HRQoL. A cardiac-specific measure of HRQoL would be less sensitive to such non-cardiac illnesses.
We used the SF–36 Physical Component Score (PCS) as the primary outcome measure, because it has greater sensitivity to change over the original eight scales of the SF–36, involves fewer statistical comparisons and eliminates floor and ceiling effects (Ware et al, Reference Ware, Kosinski and Keller1994, Reference Ware, Kosinski and Baylis1995). The PCS was derived from four sub-scales (physical function, role limitation, physical pain and health perceptions), using the method described by Ware and colleagues (Reference Ware, Kosinski and KellerWare et al, 1994). The PCS is standardised to the general population (mean score, 50: standard deviation, 10), with high scores representing higher levels of functioning. A change on the PCS of between 3 and 5 (equivalent to 0.3 to 0.5 of the standard deviation) is considered to be clinically significant (Reference Beck, Joseph and BelisleBeck et al, 2001). We did not use the mental component score as an outcome measure, as its items overlap with those of the HADS; however, we did use the SF–36 energy and vitality sub-scale as a measure of fatigue, for the purposes of the mediator analyses.
Follow-up assessment
The HADS, Illness Perceptions and SF–36 questionnaires were mailed to our study participants at 6 and 12 months following myocardial infarction. At 12 months, participants were asked whether they experienced chest pain and, if appropriate, whether they had returned to work. They were also asked to complete the Seattle Angina Questionnaire (Reference Spertus, Winder and DewhurstSpertus et al, 1995). This 19-item self-rated questionnaire assesses cardiac health status as perceived by the patient on 5 scales: physical limitations owing to chest pain, anginal stability, anginal frequency, treatment satisfaction and perceived burden of cardiac illness.
Participants were also asked to give details of hospital attendances during followup period (dates, hospitals attended and nature of health problem). Hospital attendances were verified by inspection of the appropriate hospital records, during which we confirmed whether participants had experienced any significant cardiac events during the 12 months following the myocardial infarction. These were defined as admissions to hospital or visits to accident and emergency departments with an acute cardiac problem, confirmed by investigations, e.g. chest pain associated with acute changes on echocardiogram. Both angioplasty and coronary artery bypass graft following an acute deterioration in cardiological state during the follow-up period were included.
Statistics
We used hierarchical multiple regression techniques to identify, at baseline and 6 months, variables that contributed to SF–36 PCS at 12 months after myocardial infarction, which was the dependent variable. The following independent variables were entered in blocks in the order presented below.
Block 1 (socio-demographic factors): age, gender, marital status (widowed, divorced, separated or other), education (more or less than 12 years), socio-economic status and baseline SF–36 PCS.
Block 2 (comorbid, non-cardiac illnesses): current rheumatic, respiratory, gastrointestinal or other disorder (including neurological, renal, cerebrovascular disease or endocrine disorder other than diabetes).
Block 3 (cardiac factors): ongoing risk factors for further coronary heart disease (diabetes, serum cholesterol, hypertension, previous cardiac disease, previous or current smoking v. never), measures of severity of index myocardial infarction (Killip class, CPK), site of infarction, use of thrombolysis or surgical intervention during index admission, cardiac medication on discharge and cardiac events during subsequent 12 months.
Block 4 (anxiety and depression): HADS anxiety and depression scores at baseline.
This analysis was then repeated using block 4 depression and anxiety scores recorded 6 months after infarction, in place of the same scores measured at baseline.
Collinearity diagnostics were performed and showed that, although the independent variables were associated, the degree of association was not so great as to threaten the reliability of the regression analyses (variance inflation factors all < 2.5, tolerances all > 0.4) (Reference FieldField, 2000).
In order to check that we had adjusted adequately for cardiac function, the analyses were repeated including the subgroup of 217 participants who had undergone echocardiogram, with left ventricular ejection fraction (categorised as < 30%, 30–50% and > 50%) entered into the regression equation in place of Killip class. In addition, we assessed whether chest pain, fatigue (energy and vitality sub-scale of the SF–36), illness perceptions or self-rated cardiac health status at 12-month follow-up could act as possible mediators of any association between depression/anxiety and SF–36 PCS at 12 months. These potential mediators were added after block 3, to see whether the subsequent contribution of depression/anxiety to the model was reduced, according to the method of Baron & Kenny (Reference Baron and Kenny1986).
Statistics were performed using the Statistical Package for the Social Sciences for Windows, version 11.5.0 (2002).
RESULTS
Of the 357 eligible patients, 314 (88%) gave informed consent, underwent baseline assessments and were included in the study. The mean age was 57.6 (s.d.=11.2) years and 199 (63%) were men. Site of myocardial infarction was recorded in 297, of whom 118 (37.6%) had an anterior infarction, and the majority did not have signs of cardiac failure after the infarction (Killip class I, n=230, 73.2%). On index admission, 230 patients (73.2%) received thrombolysis and 27 (8.6%) underwent a surgical intervention.
In all, 199 participants (63%) had comorbid, non-cardiac illnesses, most commonly rheumatic (32%), respiratory (15%) or gastrointestinal disorders (13%). The HADS depression score at baseline was 4.9 (s.d.=4.0), and anxiety score was 7.4 (s.d.=4.9). The mean SF–36 PCS score at baseline was 43.2 (s.d.=12.3).
Follow-up assessment
At 6 months, follow-up data were collected for 268 participants. Mean HADS depression score at 6 months was 5.6 (s.d.=4.0) and mean anxiety score was 7.7 (s.d.=4.6). Mean SF–36 PCS score was 39.0 (s.d.=11.3).
At 12-month follow-up, 16 patients had died and follow-up data were collected from 260 (83% of original sample; 87% of survivors). Participants who did not complete follow-up were significantly younger than those who did, i.e. 53.3 (s.d.=10.4) v. 57.9 (s.d.=11.3) years, respectively, more likely to have low socioeconomic status (73.7% v. 54.3%, respectively), and less likely to have received aspirin (90% v. 96%, respectively). There were no statistically significant differences in other demographic variables, medical history, severity of myocardial infarction, depression and anxiety scores or SF–36 PCS between those who completed assessments and those who dropped out of the study.
Mean HADS depression score at 12 months was 5.4 (s.d.=4.2) and mean anxiety score was 7.5 (s.d.=4.7). The mean SF–36 PCS score at 12-month follow-up was 40.4 (s.d.=11.9); participants who were depressed at this time had lower PCS scores than those who were not, i.e. mean scores=32.7 (s.d.=9.9) v. 43.8 (s.d.=11.1), respectively, t=7.8, P<0.0005.
At the time of their myocardial infarction, 137 patients were employed (part time or full time). Their mean PCS at baseline (adjusted for age and gender) was 48.7 (s.e.m.=0.86) compared with 31.8 (s.e.m.=1.9) for those on sick leave (n=29, F=43.0, P<0.0005). Of those employed at baseline, 3 died during the subsequent 12 months, and we collected complete follow-up data on 100 of the remainder (75%). For the 66 who had returned to work at 12-month follow-up, the mean PCS at follow-up (adjusted for the variables that predicted PCS at 12 months – listed in Table 1) was 46.5 (s.e.m.=1.3) and for the 30 who had not returned to work, it was 39.5 (s.e.m.=1.7, F=9.1, P=0.003).
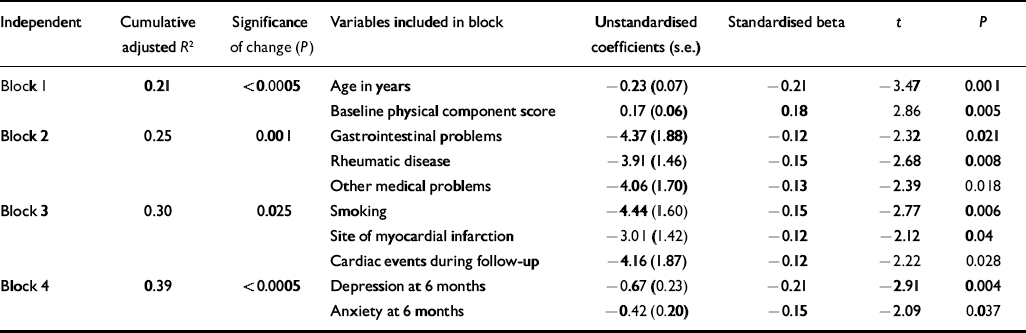
Table 1 Regression model: predictors of Medical Outcomes Study SF–36 physical component score 12 months1 after myocardial infarction
| Independent | Cumulative adjusted R 2 | Significance of change (P) | Variables included in block | Unstandardised coefficients (s.e.) | Standardised beta | t | P |
|---|---|---|---|---|---|---|---|
| Block 1 | 0.21 | <0.0005 | Age in years | -0.23 (0.07) | -0.21 | -3.47 | 0.001 |
| Baseline physical component score | 0.17 (0.06) | 0.18 | 2.86 | 0.005 | |||
| Block 2 | 0.25 | 0.001 | Gastrointestinal problems | -4.37 (1.88) | -0.12 | -2.32 | 0.021 |
| Rheumatic disease | -3.91 (1.46) | -0.15 | -2.68 | 0.008 | |||
| Other medical problems | -4.06 (1.70) | -0.13 | -2.39 | 0.018 | |||
| Block 3 | 0.30 | 0.025 | Smoking | -4.44 (1.60) | -0.15 | -2.77 | 0.006 |
| Site of myocardial infarction | -3.01 (1.42) | -0.12 | -2.12 | 0.04 | |||
| Cardiac events during follow-up | -4.16 (1.87) | -0.12 | -2.22 | 0.028 | |||
| Block 4 | 0.39 | <0.0005 | Depression at 6 months | -0.67 (0.23) | -0.21 | -2.91 | 0.004 |
| Anxiety at 6 months | -0.42 (0.20) | -0.15 | -2.09 | 0.037 |
Predictors of SF–36 PCS at 12-month follow-up
The variables that were independently associated with low SF–36 PCS at 12-month follow-up are shown in Table 1. Addition of the cardiac variables (block 3) increased the proportion of the variance accounted for (adjusted R 2) by 5%, and further addition of depression and anxiety scores at 6 months (block 4) increased the adjusted R 2 by a further 9% (F-change=10.5, P<0.0005). Anxiety and depression measured at baseline and reflecting patient mood in the weeks before first myocardial infarction did not contribute significantly to the regression model after controlling for demographic and medical details (F-change=0.89).
Repeating the above analyses using left ventricular ejection fraction in place of Killip class (n=217) did not change our findings. Depression and anxiety at 6 months continued to contribute significantly to PCS at 12 months (adjusted R 2 attributable to block 4=11%, F-change=18.6, P<0.0005).
Assessment of potential mediator variables
Reported chest pain, SF–36 energy and vitality scores, Illness Perceptions Questionnaire and Seattle Angina Questionnaire scores at 12 months were potential mediators, as they all showed significant univariate associations with both depression and anxiety at baseline and 6 months and with PCS at 12 months after myocardial infarction.
When chest pain was entered as a potential mediator, the R 2 for depression and anxiety at 6 months decreased from 9% to 5.0%, but the R 2 attributable to depression and anxiety remained significant at P<0.0005. When SF–36 energy and vitality score was added, the R 2 for depression and anxiety decreased to 0% (P=0.37). Addition of the Illness Perceptions Questionnaire sub-scores, either singly or together, reduced the R 2 that was independently attributable to the depression and anxiety measures, although in all models the contributions of depression and anxiety remained significant (P≤0.01). Similarly, addition of the scores from the Seattle Angina Questionnaire reduced the magnitude of the R 2 attributable to anxiety and depression, although it remained significant (all P≤0.002).
The effects of changing depression on SF–36 PCS
To clarify the effect of depression severity changing between baseline and 12-month follow-up, we compared changes in the SF–36 PCS of 38 participants who were not depressed at baseline but who became depressed during follow-up, with those of 143 participants who remained undepressed throughout the study period, using HADS total of ≥17 to indicate depression. The HADS depression scores of these two groups at baseline were 3.2 (2.2) and 2.7 (2.5), respectively, (t=–1.3, P=0.2), confirming the non-depressed status of both groups at baseline. There was no significant difference in SF–36 PCS at baseline between these two groups: scores were 42.1 (12.8) and 45.5 (11.5), respectively (t=1.6, P=0.12). The HADS depression score at 12 months for individuals who became depressed was 10.0 (3.2) whereas for those who remained nondepressed it was 3.0 (2.2), (t=–15.6, P<0.0005). The SF–36 PCS for these groups were 30.9 (7.4) and 44.1 (10.7) (t=8.8, P<0.0005).
DISCUSSION
We have shown that depression and anxiety recorded 6 months after first myocardial infarction predicted impairment in the physical aspects of HRQoL at 12 months after the infarction. This adverse impact of depression is additional to the effect on HRQoL of the myocardial infarction itself, ongoing angina and cardiac events during the follow-up period. Depression and anxiety in the period immediately preceding the first myocardial infarction did not predict the physical aspects of HRQoL 12 months later, however. Thus our hypothesis was partly proved.
Our method has a number of strengths, according to the quality criteria used in the systematic review of Sørensenf et al (Reference Sørensenf, Friis-Hashe and Haghfelt2005). First, we screened a consecutive series of patients presenting with first myocardial infarction, and managed to recruit and follow up a high proportion of eligible individuals. Second, we included only people who had not experienced previous myocardial infarction, to avoid any confounding effect of previous infarction on the association between depression and later impairment of HRQoL. Third, we used valid and widely recognised measures of depressive symptoms and HRQoL that were appropriate for our population, and we used the same measures over three waves of assessment. In our regression analyses, we controlled for a wider range of potential confounders than any previous study, including the effects of cardiac and non-cardiac illness, and for baseline SF–36 PCS before assessing the contribution of anxiety and depression. Furthermore, we assessed rigorously the cardiac status of participants, particularly with regard to the severity of myocardial infarction, using a range of clinical and laboratory techniques. Thus we can be confident that our assessment of the contribution of anxiety and depression to subsequent HRQoL was conservative.
Our study has some weaknesses. Our assessments of depression and HRQoL were retrospective, making them vulnerable to recall bias. However, we had validated the HADS administered in this way against a standardised psychiatric interview that asked in detail about each depressive symptom (sleep loss, appetite difficulty, mood, etc.) for the month before the myocardial infarction (Reference Dickens, McGowan and PercivalDickens et al, 2004b ). We were also able to show the validity of the SF–36 in relation to employment status. Furthermore, our findings are supported by those of a truly prospective study that showed that onset of coronary heart disease led to deterioration of physical function and role performance, when it was associated with increased anxiety and depression (Reference van Jaarsveld, Sanderman and Ranchorvan Jaarsveld et al, 2002).
Psychiatrists will be familiar with the impairment in the mental component of the SF–36 that occurs with depression, but we have shown that when depression is concurrent with physical illness there is also a reduction of the physical component. The low SF–36 PCS score of participants who were depressed at 12 months represented very marked impairment, with a mean score one standard deviation lower (more impaired) than that for non-depressed participants and nearly two standard deviations lower than the score for the general population.
We have shown previously that fatigue is associated with depression in patients presenting with myocardial infarction (Reference McGowan, Dickens and PercivalMcGowan et al, 2004). This current report indicates that this fatigue mediated the observed association between depression and HRQoL. There are a number of mechanisms by which fatigue might be associated with impairment in HRQoL. Fatigue may occur as the direct result of depression, and may be mistaken for a symptom of ongoing cardiac problems, thus leading to impaired HRQoL by virtue of increasing symptom load. Furthermore, fatigue following myocardial infarction may lead to deconditioning (or possibly, failure to rehabilitate) through reduced tolerance of physical activity, with consequent loss of physical function (Reference Lavoie, Fleet and LesperanceLavoie et al, 2004).
It is interesting that depression and anxiety that preceded myocardial infarction did not predict impairment in HRQoL 12 months following infarction, whereas depression and anxiety recorded 6 months after infarction did so. We have shown previously, however, that there are considerable changes in depression scores over the period following myocardial infarction; 45% of those depressed at baseline improved during the 12 months following infarction, whereas 21% of those not depressed at baseline developed depression during follow-up (Reference Dickens, Percival and McGowanDickens et al, 2004a ). It is possible that these changes in depression reduced the effect of pre-infarction depression on HRQoL 12 months after infarction in our regression analyses.
Our findings have considerable implications for clinical practice. Although depression is common following myocardial infarction, the people recruited for our study very rarely attracted treatment. This may represent hesitancy on the part of general practitioners and other doctors to prescribe antidepressants after myocardial infarction, even though this is generally safe (Reference Glassman, O'Connor and CaliffGlassman et al, 2002). It is more likely that depression is rarely recognised and treated in the physically ill, even though such treatment is effective (Reference Gill and HatcherGill & Hatcher, 1999).
Psychiatrists should be prepared to treat, or advise general practitioners to treat, depression using antidepressant and cognitive–behavioural therapy. Our findings indicate the importance of detecting and treating depression and anxiety, since it would be expected that reducing depression and anxiety would lead to improved quality of life and reduction in days of restricted activity and days missed from work (Reference Kisely and SimonKisely & Simon, 2005). This remains to be demonstrated in a clinical trial, however (Reference Simon, Katon and RutterSimon et al, 1998).
Acknowledgements
This study was funded by the Medical Research Council of the UK and the British Heart Foundation.






eLetters
No eLetters have been published for this article.