Brazilian dietary habits are in the process of nutritional transition(Reference Batista Filho and Rissin1). A decrease in the consumption of naturally and minimally processed foods, such as rice, beans, fruit and vegetables, and an increase in the consumption of ultra-processed foods, rich in oils, sugars and synthetic substances, such as soft drinks, biscuits, processed meats and instant pasta have been noticed (Reference Monteiro, Levy and Claro2).
The NOVA system was created to classify foods based on the extent and degree of processing used in their products into four groups: unprocessed or minimally processed foods (group 1), processed culinary ingredients (group 2), processed foods (group 3) and ultra-processed food and drink products (group 4)(Reference Monteiro, Cannon and Levy3). NOVA is a valid tool for nutrition and public health and has been recognised by the Brazilian Ministry of Health and international organisations such as the Food and Agriculture Organisation of the United Nations and the Pan American Health Organisation(Reference Monteiro, Cannon and Levy3). As these products are easily accessible and prepared, ready or pre-prepared for consumption and attractive to the palate, the consumption of foods from the ultra-processed group has become increasingly present in the food routine of the population in Brazil and worldwide(Reference Monteiro, Moubarac and Cannon4). Moreover, ultra-processed foods have been produced at very low cost and heavily marketed, particularly to low-income families(Reference Martins, Levy and Claro5).
Interest in research to monitor the consumption of ultra-processed foods in the population, as well as their association with health outcomes is growing. Consumed at high frequency, these foods can contribute to the increased prevalence of chronic non-communicable diseases and conditions in childhood and adolescence, such as obesity(Reference Costa, Del-Ponte and Assuncao6) and cardiovascular problems(Reference Rauber, Campagnolo and Hoffman7). Childhood is a critical stage in the acquisition of eating behaviours and exposure to future health problems and illnesses(Reference Craigie, Lake and Kelly8), making it relevant to prioritise investigations in this period of life.
While the topic of ultra-processed foods consumption has been explored for various health outcomes(Reference Monteiro, Cannon and Lawrence9), its role in oral health is not well known. Although the causal relationship between sugar and dental caries is already well established(Reference Touger-Decker and van Loveren10–Reference Sheiham and James12), most studies have investigated only foods that contain sucrose in their formulation(Reference Moynihan and Kelly13), which does not include some ultra-processed foods. Current evidence indicates that sucrose is not the only carbohydrate with cariogenic potential, meaning that it could cause caries, and that foods containing other types of fermentable carbohydrates tend to be retained in the teeth for a longer period, contributing to the development of the disease(Reference Gupta, Gupta and Pawar14,Reference Bradshaw and Lynch15) .
Dental caries is the most prevalent chronic disease in preschool children in the world(Reference Marcenes, Kassebaum and Bernabe16) and has serious negative impacts on quality of life(Reference Haag, Peres and Balasubramanian17) as well as high treatment costs for the family and society(Reference Listl, Galloway and Mossey18). The disease is caused by the complex interaction of socio-economic risk factors (e.g., low maternal educational attainment and low household income) with low access to and use of preventive health services, which increase the likelihood of risky behaviours such as high consumption of cariogenic foods and irregular oral hygiene(Reference Selwitz, Ismail and Pitts19).
The new Dietary Guidelines for Brazilian Children Under Two Years of Age, published in 2019(20), recognises the relationship between ultra-processed foods and caries and recommends the non-consumption of these foods before the age of 2 years. After this age, the diet should be essentially based on unprocessed or minimally processed foods with limited consumption of processed and ultra-processed foods(21). However, to our knowledge, the consumption of ultra-processed foods, according to the definition of the NOVA system, has not been explored in relation to childhood oral health outcomes. From the public health practical point of view, it is useful to explore the influence of consumption of ultra-processed foods on oral health, focusing at producing evidence to be translated into interdisciplinary and multi-sectoral population interventions(Reference Leme, Fisberg and Thompson22). The current study aimed to investigate the relationship between the daily frequency of ultra-processed food consumption and the presence of caries in children from 0 to 3 years of age, registered in Primary Healthcare Centers (PHC) in Brazil. The hypothesis tested in the study is that the consumption of ultra-processed foods four times or more a day is associated with early childhood caries and that child age modifies this effect.
Methods
Study population and design
This is a cross-sectional analysis of baseline data from a cluster randomised controlled trial, conducted in the urban area of the municipality of Pelotas, Southern Brazil (Brazilian Clinical Trial Registry protocol number RBR-74jbmn)(Reference Cascaes, Menegaz and Quevedo23). The baseline was conducted between September and December 2015.
Pelotas has 329 435 inhabitants, 22 500 children, from 0 to 5 years old, predominantly living in the urban area, with access to public fluoridated water supply(24). In 2015, the Public Health System of Pelotas was composed of a total of twenty-four PHC offering Family Health Teams in the urban area, and eight of these had Oral Health Teams. Of the total of eight eligible PHC, four were included in the trial because they had the following characteristics: (i) belonged to the urban area, (ii) had a full-time Oral Health Team working at the PHC for at least 1 year, (iii) had a minimum of 200 children aged 0–5 years registered at the PHC in the last year and (iv) offered perinatal care and childcare. Four PHC were excluded for presenting: (i) non-comparable socio-economic and health indicators, (ii) other oral or nutritional health interventions in progress and (iii) a differentiated work process, such as having a dental hygienist on the team.
The sample calculation was based on the fixed number of two clusters available for each group and seventy-five children per cluster (n 300), with an intra-class correlation coefficient of 0·05, a power of 80 % and significance level of 0·05 to detect minimum differences between groups ranging from 12 to 16 %(Reference Hemming, Girling and Sitch25). For the final sample, 30 % was added to cover losses and rejections throughout the study (n 390). The children were randomly selected in proportion to the number and age of enrolled children in each micro area of the PHC territories. Only children who had at least one tooth (n 309) were included in the current study.
Data collection was carried out in the children’s homes by a team composed of sixteen interviewers and two dentists, accompanied by the community health worker responsible for each micro area. The caregivers answered a questionnaire and the children received an epidemiological oral examination for caries evaluation. The team received theoretical and practical training to conduct interviews and examinations. The questionnaire was pretested with a sample of caregivers of children with the same age as the target population for comprehension and language adjustment. Dentists were calibrated for the examination of tooth surfaces using the gold standard examiner method. Excellent agreement was obtained with the gold standard for assessing caries in children (minimum κ of 0·90). Detailed information about the study methodology can be found in another publication(Reference Cascaes, Menegaz and Quevedo23).
Oral health assessment (main outcome)
Early childhood caries was defined as an outcome of the study and was evaluated separately by the presence of non-cavitated caries (active and inactive white spots) and cavitated caries using the decayed, missing and filled surfaces due to caries index (dmf-s index). The criteria for evaluating non-cavitated caries considered the presence of stains located at the level of tooth enamel, in areas of stagnation of dental biofilm (cervical, occlusal and proximal). Non-cavitated caries was classified as inactive when intact stains presented shiny and smooth, white, yellowish or brownish appearance and was classified as active when intact stains presented white, opaque or rough appearance. Cavitated caries was evaluated according to the WHO criteria and recommendations(26). Both outcomes were dichotomised in the absence and presence of children with caries.
Ultra-processed food consumption (main exposure)
The daily frequency of ultra-processed food consumption was investigated using an FFQ, containing thirty-five items answered by the caregiver. This FFQ was adapted from similar instruments previously used in studies of the Pelotas birth cohorts(Reference Bielemann, Motta and Minten27,Reference Peres, Sheiham and Liu28) and the list of food consumption markers in Brazil(29). The Pelotas birth cohorts assessed all foods as single items (e.g., list of different types of fruit). We grouped some items into an overall group that represents that type of food (e.g., ‘fruit or fruit salad’), such as the healthy and unhealthy eating markers for the Brazilian population(29). Ultra-processed foods items were detailed in our FFQ, given that these were the exposures of interest to our study. The period that the FFQ refers to is the last 30 days. It evaluated the number of times each ultra-processed food was consumed, as well as its periodicity: daily, weekly or monthly, with a continuous scale. The amount or portion size was not asked. The ultra-processed foods investigated accounted for fifteen items: chocolate powder, dairy sweetened beverage, artificial juice, canned fruit juice, instant noodles, salty crackers, sweet-filled biscuit/cracker, snacks (chips), hamburgers and sausages, chocolate bars/bonbons, candies/caramels, gum/lollypop, regular sodas, light soft drinks, gelatin, and ice cream/popsicle. These items meet the criteria for ultra-processed foods according to the NOVA Food Classification System(Reference Monteiro, Cannon and Levy3): ‘industrial formulations made entirely or mostly from substances extracted from foods (oils, fats, sugar, starch, and proteins), derived from food constituents (hydrogenated fats and modified starch), or synthesized in laboratories from food substrates or other organic sources (flavour enhancers, colours, and several food additives used to make the product hyper-palatable)’.
The ultra-processed food consumption frequencies were added together and transformed into the daily unit, categorising total daily consumption into (i) up to three times and (ii) four times or more. This categorisation was chosen for two reasons. Initially, the intention was to use the variable in four categories of consumption: not consumed, consumed 1–2 times a day, consumed three times a day or consumed four times or more a day. However, there were no children with caries in the ‘did not consume’ and ‘consumed 1–2 times a day’ categories, which made it impossible to perform adjusted analyses. Thus, the dichotomous categorisation (up to three times v. four times or more) was established based on evidence from oral health promotion studies, which discuss an increased risk for caries in children when foods containing sucrose are consumed four times or more a day(30).
Covariates and potential confounders
Socio-demographic characteristics of the caregivers/families and children, as well as oral hygiene and use of dental services by the children, were assessed as covariates and potential confounding factors. The caregiver/family socio-demographic variables were degree of kinship with the children (mother or others), caregiver age (16–29, 20–44 and 45 or more years), caregiver marital status (married or living with a partner; single or without a partner), caregiver educational attainment (0–4, 5–8 and 9 or more years of study) and per capita family income. The socio-demographic variables for children were gender (female and male), child age (≤2 or >2 to 3 years), skin colour (white and black/brown) and breast-feeding status (mean time in months).
Child oral hygiene was considered the age at which the child started brushing/cleaning his/her teeth (at 12 months or earlier, after 12 months and never brushed/cleaned). Child use of dental services was characterised by having been to the dentist or not, together with the reason for the last visit, categorised into: have been to the dentist for routine or prevention purposes, have been to the dentist for pain or treatment and have never been to the dentist.
Statistical analyses
The data were analysed using the Stata/IC 14.2 statistical programme. Initially, a descriptive analysis was performed, presenting the absolute and relative frequencies of the categorical variables and the average of total and individual daily consumption of ultra-processed foods in children with no caries, non-cavitated caries (white spots) and cavitated caries (dmf-s index). Bivariate and adjusted Poisson regression analyses were conducted to examine the association between daily consumption of ultra-processed foods and the presence of non-cavitated caries (white spots) and cavitated caries (dmf-s index). The analyses used robust variance adjustment, obtaining prevalence ratios (PR) and their respective 95 % CI. The robust variance fit can be considered an adjustment for overdispersion of zeros, something common in cross sectional studies with binary outcomes with great inequality in distribution, such as dental caries(Reference Barros and Hirakata31). The variables for fitting were added at three levels. On the first level, socio-demographic variables related to the caregiver/family were included. On the second level, demographic variables related to the child and breast-feeding status were included. Finally, variables related to child oral hygiene and use of dental services were included. Only variables with P < 0·20 in the bivariate analysis were considered as potential confounding variables of the relationship between daily consumption of ultra-processed foods and caries and, therefore, were included in the model for adjustment. The final model considered the value of P < 0·05 to identify the independent association of the daily consumption of ultra-processed foods with the analysed outcomes. The interaction between child age and daily ultra-processed food consumption on the occurrence of early childhood caries was estimated. The adjusted prevalence of non-cavitated caries (white spots) and cavitated caries (dmf-s index) was presented according to different combinations between child age and daily ultra-processed food consumption.
Results
Table 1 presents the distribution of the sample and the outcomes concerning the socio-economic and demographic characteristics of the participants and children’s behaviours. The majority of the children’s caregivers were mothers (79·0 %), aged between 16 and 29 years (52·4 %), married or living with a partner (87·7 %) and with 9 or more years of schooling (52·8 %). Per capita family income of the richest tercile was on average R$ 742·89 (equivalent to $US 187·59). Regarding children, 53·4 % were girls and 81·6 % were of white skin colour. Average duration of breast-feeding was 14·6 (sd 14) months. More than half (62·0 %) started brushing/cleaning their teeth at 12 months or earlier. On the other hand, 71·2 % had never been to the dentist and 17·2 % had been to the dentist for routine or prevention purposes. Total daily consumption of ultra-processed foods four times or more was found in 67·6 % of the children.
Table 1 Sample and outcomes distribution according to caregiver/family and child socio-economic and demographic characteristics and child health behaviours (n 309) (Pelotas/RS, Brazil, 2015)

* P-value refers to Wald’s test.
† Variable with twelve (family income), five (caregiver education) and one (age started to brush/clean your teeth) missing data.
In the oral examination, 20·4 % of children presented non-cavitated caries (white spots) and 9·4 % had cavitated caries (dmf-s index). The prevalence of non-cavitated caries (white spots) and cavitated caries (dmf-s index) was 24·4 and 12·0 %, respectively, in the group of children with total daily consumption of ultra-processed foods four times or more (Table 1).
Figure 1 describes the average daily consumption of each ultra-processed food among children without caries, with non-cavitated caries (white spot) and with cavitated caries (dmf-s index). The average daily consumption of ultra-processed foods was higher in children with cavitated caries compared with those with non-cavitated caries and without caries, except for gelatin, chocolate powder and sweet-filled biscuit/cracker. Similar findings were observed for children with non-cavitated caries (white spots) in relation to children with no caries, except for sweet-filled biscuit/cracker. The most frequently consumed ultra-processed foods were chocolate powder, dairy sweetened beverage and salty crackers. Light soft drinks and ice cream/popsicle were the foods with the lowest average consumption in all groups.
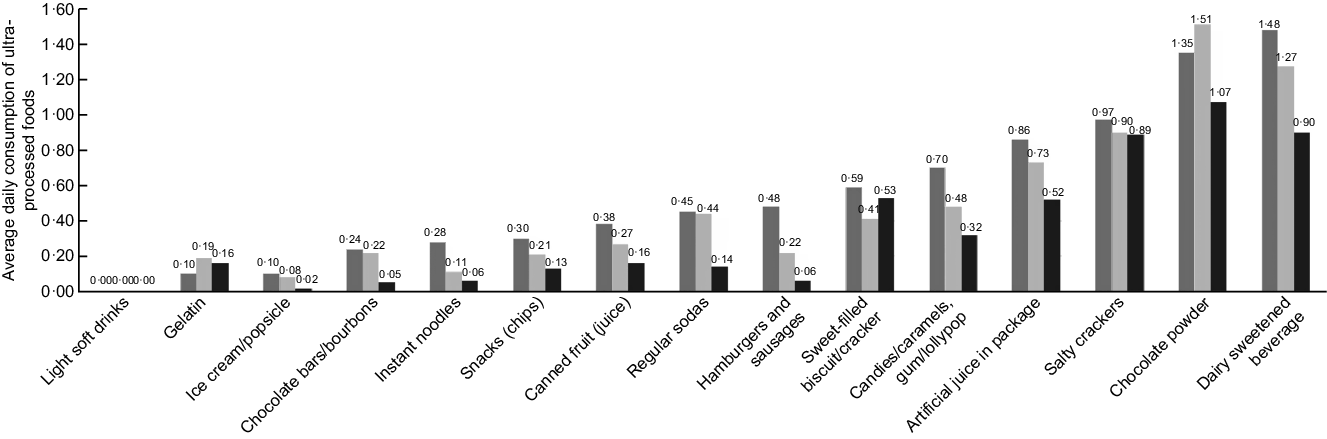
Fig. 1 Average daily consumption of ultra-processed foods in children aged 0–3 years without caries (![]() ), with non-cavitated caries (white spot,
), with non-cavitated caries (white spot, ![]() ) and with cavitated caries (dmf-s index,
) and with cavitated caries (dmf-s index, ![]() ) (n 309) (Pelotas/RS, Brazil, 2015)
) (n 309) (Pelotas/RS, Brazil, 2015)
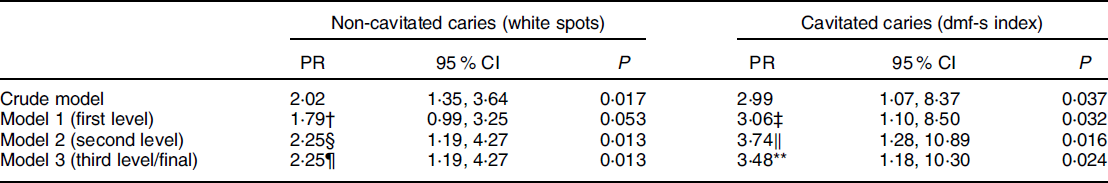
In the bivariate analysis, children who consumed ultra-processed foods four times or more a day had an increased probability approximately two times greater of having non-cavitated caries (PR 2·02, 95 % CI 1·35, 3·64) and three times greater of having cavitated caries (PR 2·99, 95 % CI 1·07, 8·37), compared with those who consumed ultra-processed foods up to three times a day (Table 2). After adjustment for confounding, these associations remained similar. Children who consumed ultra-processed foods four times or more a day had approximately twice as much risk of presenting non-cavitated caries (PR 2·25, 95 % CI 1·19, 4·27) and approximately three times as much risk of cavitated caries (PR 3·48, 95 % CI 1·18, 11·30) compared with those who consumed them three times or less a day (Table 2).
Table 2 Crude and adjusted Poisson regression analysis between the daily ultra-processed food consumption and the presence of non-cavitated caries (white spots) and cavitated caries (dmf-s index) in children aged 0–3 years (n 309) (Pelotas/RS, Brazil, 2015)*
PR, prevalence ratio.
* Daily ultra-processed food consumption (0 = up to three times and 1 = four times or more).
† Adjusted for caregiver educational attainment.
‡ Adjusted for caregiver age.
§ Adjusted for caregiver educational attainment and duration of breast-feeding.
‖ Adjusted for caregiver age and duration of breast-feeding.
¶ Adjusted for caregiver educational attainment and duration of breast-feeding.
** Adjusted for caregiver age, duration of breast-feeding and dental service utilisation.
A significant interaction between child age and daily ultra-processed food consumption was found for non-cavitated caries (white spots) (P interaction = 0·012). Although this interaction was not significant for cavitated caries (dmf-s index) (P interaction = 0·381), the group of children aged >2 to 3 years with a daily frequency of four times or more of ultra-processed foods had the highest adjusted prevalence of both outcomes (Fig. 2).
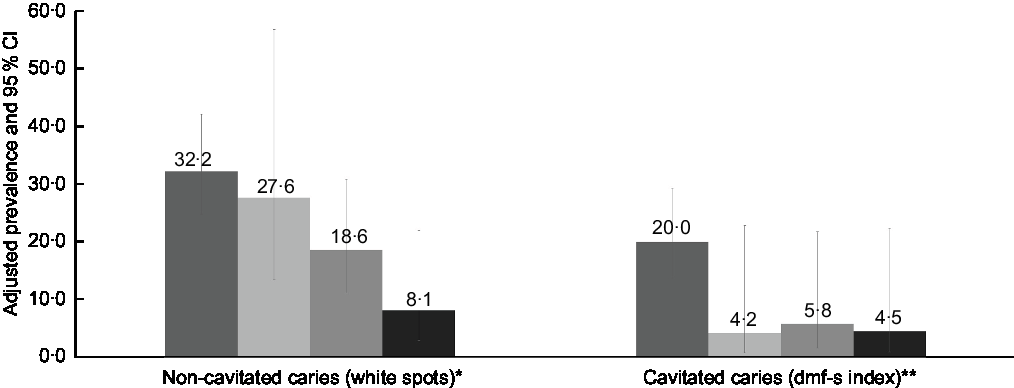
Fig. 2 Adjusted prevalence of non-cavitated caries (white spots) and cavitated caries (dmf-s index) according to the interaction between child age (≤2 and >2 to 3 years) and daily ultra-processed food consumption (up to three times and four times or more) in children aged 0–3 years old (n 309) (Pelotas/RS, Brazil, 2015). *Adjusted for caregiver educational attainment and duration of breast-feeding; P
interaction effect = 0·012. **Adjusted for caregiver age, duration of breast-feeding and dental service utilisation. P
interaction effect = 0·381. ![]() , children aged >2 to 3 years with daily consumption of ultra-processed food four times or more (n 123);
, children aged >2 to 3 years with daily consumption of ultra-processed food four times or more (n 123); ![]() , children aged ≤2 years with daily consumption of ultra-processed food four times or more (n 27);
, children aged ≤2 years with daily consumption of ultra-processed food four times or more (n 27); ![]() , children aged >2 to 3 years with daily consumption of ultra-processed-foods up to three times (n 86);
, children aged >2 to 3 years with daily consumption of ultra-processed-foods up to three times (n 86); ![]() , children aged ≤2 years with daily consumption of ultra-processed-foods up to three times (n 73)
, children aged ≤2 years with daily consumption of ultra-processed-foods up to three times (n 73)
Discussion
To our knowledge, this is the first study to explore the association between ultra-processed foods and oral health based on the new definition proposed by the NOVA system. The current study showed that approximately two-thirds of children from 0 to 3 years of age consumed ultra-processed foods four times or more a day, and that this consumption is related to an increased probability of presenting caries in the first years of life. This relationship was stronger among children aged >2 to 3 years. PR and average daily consumption of ultra-processed foods were higher among children who had cavitated caries compared with those with non-cavitated caries, indicating a possible relationship between the dosage of these foods and the severity of the disease.
Previous studies carried out with Brazilian preschoolers also identify a high frequency of ultra-processed food consumption, characterising a deterioration of dietary habits in early childhood in different regions in the country. In São Luis Maranhão, Northern Brazil, it was estimated that 25 % of the total energy intake in a sample of children aged 13–35 months came from ultra-processed foods, and this consumption was higher among those over 16 months(Reference Batalha, Franca and Conceição32). A study conducted with a sample of children aged 2–9 years in Campinas, São Paulo, Southeastern Brazil, showed that consumption of ultra-processed foods was predominant (66 %)(Reference Mais, Warkentin and Vega33). In São Leopoldo, Rio Grande do Sul, Southern Brazil, a survey revealed that among 4-year-old children ultra-processed foods represented 42 % of the total energy consumed(Reference Costa, Rauber and Leffa34). Direct comparisons between these studies and the present one should be made with caution due to differences in children’ age, dietary methods applied to data collection and classification of ultra-processed foods.
In the current research, the most consumed ultra-processed foods were dairy sweetened beverage and chocolate powder, the latter possibly added to milk in bottles, a common eating behaviour in children of this age group. Many caregivers still believe that these foods are nutritious and healthy, which is why they might offer them to their children. Foods containing more than 5 g of sugars per serving are considered highly cariogenic and when in frequent contact with the oral cavity can cause caries(30). High amount of sugars is a characteristic of many dairy sweetened beverages, chocolate powder and chocolate milk beverages sold in Brazil. Evidence indicates that frequent daily consumption of potentially cariogenic foods is associated with increased occurrence of caries in preschool children(Reference Feldens, Rodrigues and de Anastacio35,Reference Park, Lin and Onufrak36) , a finding that corroborates the findings of the current research. It has been well documented in the literature that public fluoridated water reduces the prevalence of caries at all ages(Reference McDonagh, Whiting and Wilson37,Reference Do, Miller and Phelan38) . Given that our study population has access to fluoridated public water, we speculate that such findings could be even worse in those living in non-fluoridated areas.
Eating behaviours acquired early in life can influence oral health in a contemporary and prospective manner(Reference Peres, Sheiham and Liu28,Reference Feldens, Rodrigues and de Anastacio35) . In turn, consequences of caries, such as pain and tooth loss, are associated with difficulties in chewing and sleeping, worsening in the quality of diet and nutritional profile of individuals(Reference Psoter, Reid and Katz39,Reference So, Ellenikiotis and Husby40) . Nutritional deficiencies can also cause various forms of damage to oral structures and tissues, generating a series of manifestations in the oral cavity(Reference Pflipsen and Zenchenko41). This evidence indicates the close relationship between diet and oral health, which reinforces the importance of studies in this issue.
The negative effect of cariogenic food consumption on oral health can be reduced through good oral hygiene practices and regular use of preventive dental services. Our findings indicate that the association between consumption of ultra-processed foods and caries remained significant independent of oral hygiene and preventive use of dental services. A longitudinal analysis of the 1993 Pelotas Birth Cohort study revealed that the caries lifetime increase was 66 % higher in the group of children with a high frequency of sugar consumption compared with those with low consumption, regardless of the use of dental services and regular oral hygiene(Reference Peres, Sheiham and Liu28). These results highlight the potential that effective interventions to change eating behaviours can have on children’s oral health outcomes, corroborating the argument of experts(Reference Schulte and Tsakos42).
Some limitations of the current study should be discussed. One of them is the cross-sectional aspect of the analyses, which limits investigating the causal relationship of the associations obtained. This is not a population-based study and may underestimate the contribution of some risk factors. For example, the absence of some socio-economic factor effects, which are commonly associated with dental caries and food consumption patterns, may be due to the homogeneity of the sample as a result of the randomisation process. The sample was composed of children belonging to low-income families and users of PHC, which makes it difficult to generalise the findings to the Brazilian population who do not use the public health sector primary care. In the absence of a validated FFQ for the population from 0 to 3 years of age, an instrument was developed for the current study. Our FFQ did not include some foods that might be frequently consumed by children under the age of 3 years in developed countries (e.g., instant flours, sugary breakfast cereals and industrialised cakes). This may limit direct comparison with other studies across countries. In addition, no quantification of food consumed was evaluated, limiting the investigation of the relationship between ultra-processed food consumption quantity and dental caries. However, for oral health, the frequency of consumption of cariogenic foods is more important than the size of the portions consumed, since it is the frequent contact of the fermentable carbohydrate with the oral cavity that increases the risk for the disease(Reference Feldens, Rodrigues and de Anastacio35). In the current study, it was not possible to compare non-consumption of ultra-processed foods v. categorisation from once-daily consumption, since no children who consumed ultra-processed foods up to twice a day presented caries. The vast majority of our children do not attend daycare centres, but in some situations, their care is shared by family members, such as grandparents or older siblings. This could have added another limiting aspect as the child’s diet was mentioned only by the main caregiver. However, we believe this is not a relevant limitation, as, at the age of the children surveyed, the majority of the caregivers responding are primarily responsible for the food provided to the child. Despite these limitations, our study has important methodological strengths, such as a trained team, standardised instruments for data collection and assessment of outcomes.
Conclusion
The current study indicates a positive relationship between the daily frequency of ultra-processed food consumption and the prevalence of caries in early childhood. The findings reinforce the importance of implementing interdisciplinary and multi-sectoral interventions aimed at promoting children’s healthy eating and oral health. Such interventions should include maternal−child health programmes to provide nutrition and oral health education for caregivers and families about avoiding feeding young children ultra-processed foods in combination with public policies to limit the marketing and consumption of these foods in early childhood. Longitudinal analyses from this and other studies are suggested to broaden the understanding of the influence of ultra-processed foods on children’s oral health. Future studies exploring the dose−response relationship in terms of frequency consumption of ultra-processed foods and oral health are suggested.
Acknowledgements
Acknowledgements: The authors thank the Department of Primary Healthcare of the Federal University of Pelotas and all volunteer research assistants who were involved in the current study. The authors specially thank all parents and children who took part of the current study. Financial support: The study data collection was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil − Finance Code 001 and data analyses by The National Council for Scientific and Technological Development (CNPq Process no. 421044/2018-7; Notice MCTI/CNPQ/Universal 28/2018). Conflict of interest: There are no conflicts of interest. Authorship: A.M.C. and M.S.S. designed the study protocol. A.M.C., J.d.S.V. and T.M.S. conducted the statistical analysis. M.S.S and A.M.C. wrote the first draft. A.M.C., J.d.S.V., T.M.S. and R.A.B. provided critical review. All authors reviewed and commented on subsequent drafts and approved the final manuscript. Ethics of human subject participation: The current study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving research study participants were approved by the Research Ethics Committee of the Federal University of Pelotas School of Medicine (protocol no. 1.206.247). Written informed consent was obtained from all caregivers.









