Metabolic syndrome (MetS) is a cluster of interrelated cardiovascular risk factors including obesity, dyslipidaemia, hypertension and high blood glucose (with or without diabetes)( Reference Alberti, Eckel and Grudy 1 ) that is highly prevalent worldwide( Reference Cornier, Dabellea and Hernandez 2 ) and is associated with substantial social and economic costs( Reference Kassi, Pervanidou and Kaltsas 3 ). The prevalence of MetS has increased in particular in low- and middle-income countries( Reference Misra and Khurana 4 ) which have or had a high prevalence of child malnutrition( Reference Black, Victora and Bhutta 5 , Reference Doak, Adair and Bentley 6 ).
According to the concept of fetal origins of adult disease, fetal adaptation to nutrient restriction in the uterus induces permanent changes in organ and tissue function, leading to increased risk of cardiometabolic diseases in adult life( Reference Barker 7 – Reference Hales and Barker 9 ). Low birth weight (LBW), a robust marker of intra-uterine nutritional deficiency, is associated with MetS in adults regardless of potential confounding factors( Reference Jong, Lafeber and Cranendonka 10 – Reference Barker, Hales and Fall 12 ). Previous findings also suggest that individuals with LBW and higher BMI at the age of 20 years are more likely to have insulin resistance( Reference Yeung 13 ), CHD and type 2 diabetes( Reference Eriksson 14 ) than non-overweight individuals of the same age.
Leg length is another factor that is heavily influenced by nutritional conditions during childhood, especially until the age of 7 years( Reference Azcorra, Varela-silva and Rodriguez 15 ). Several studies have described statistically significant associations between shorter relative leg length and distinct components of MetS in adults, including type 2 diabetes( Reference Mueller, Duncan and Barreto 16 , Reference Weitzman, Wang and Pankow 17 ), hypertension( Reference Langenberg, Hardy and Breeze 18 ) and hypertriacylglycerolaemia( Reference Smith, Greenwood and Gunnell 19 ), as well as with metabolic alterations like lower insulin sensitivity( Reference Johnston, Harris and Retnakaran 20 ). Furthermore, associations between lower leg length and MetS have been reported in children and older adults( Reference Liu, Liu and Li 21 , Reference Pryzbek and Liu 22 ). Although LBW and shorter relative leg length are correlated, evidence shows that the two measures are complementary and that they are to some extent independent consequences of the pre- and postnatal environments, respectively( Reference Bogin and Baker 23 ).
Some evidence indicates that the association of nutritional deprivation in utero with adulthood health outcomes such as obesity varies according to gender( Reference Imai, Halldorsson and Gunnarsdottir 24 , Reference Ravelli, Meulen and Osmond 25 ). Previous analysis from the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil) has endorsed this latter finding( Reference Yarmolinsky, Mueller and Duncan 26 ). However, most studies that investigated the relationship between nutritional deprivation and MetS did not perform gender-stratified analyses. Nevertheless, we believe that the investigation of nutritional deprivation and MetS should be stratified by gender because: (i) body proportions vary according to sex( Reference Bogin and Varela-Silva 27 ); (ii) the association of short stature with adiposity, specifically central obesity, is observed only in women, not in men( Reference Velasquez-Melendez, Siveira and Allencastro-Souza 28 ); and (iii) earlier age at menarche is associated with shorter leg length( Reference McIntyre 29 , Reference Onland-Moret, Peeters and Gils 30 ) and increased chances of diabetes and cardiometabolic disease, reinforcing the need to perform gender-stratified analysis( Reference Mueller, Duncan and Barreto 31 ).
Studies concerning early-life adversity and chronic non-communicable diseases in adult life remain scarce in low- and middle-income countries, such as Brazil. ELSA-Brasil is a cohort of 15 105 civil servants born between 1934 and 1975, a period when child malnutrition was common in Brazil( Reference Schmidt, Duncan and Mill 32 ). After a rapid nutritional transition, obesity increased significantly in Brazil, creating an opportunity to investigate the effects of adverse early-life nutritional conditions on adult health( Reference Conde and Monteiro 33 ).
The present study investigated gender-specific associations of LBW and shorter relative leg length with a higher prevalence of MetS after adjusting for sociodemographic characteristics and health-related behaviours. In addition, we determined whether these associations remained significant after adjusting for age at menarche (in women) and BMI at 20 years old, as markers of malnutrition also affect these factors during childhood. We hypothesized that LBW and shorter relative leg length would be associated with a higher prevalence of MetS in adults, even after adjusting for BMI at 20 years old and age at menarche.
Methods
Study population
A cross-sectional analysis was conducted using baseline data from ELSA-Brasil (2008–2010). ELSA-Brasil is a multicentre cohort of 15 105 civil servants, aged 35 to 74 years, from universities and research institutions located in six Brazilian cities( Reference Schmidt, Duncan and Mill 32 , Reference Aquino, Barreto and Benseno 34 ). Baseline data were collected via face-to-face interviews, medical examinations and laboratory tests conducted by trained and certified professionals. All procedures were standardized and data collection instruments pre-tested in pilot studies. The Ethics and Research Committees of each institution approved the ELSA-Brasil research protocol. All participants signed an informed consent form.
The current analysis excluded 1030 participants who had at least one self-reported CVD (myocardial infarction, cerebrovascular accident, revascularization or cardiac insufficiency), premature birth (n 712) and individuals who described their race as Asian (n 322) or indigenous (n 129), for the following reasons: (i) CVD is itself a condition related to LBW, lower leg length( Reference Ferrie, Langenberg and Shipley 35 ) and MetS( Reference Galassi, Reynolds and He 36 ); (ii) premature birth often results in LBW due to gestational circumstances other than nutritional factors( Reference Offenbacher, Katz and Fertik 37 ); and (iii) different cut-off points for waist circumference are recommended for Asian and indigenous individuals( Reference Alberti, Eckel and Grudy 1 ). We also excluded participants who had missing data on: (i) self-reported race (n 148); (ii) any of the MetS criteria (n 105); (iii) age at menarche (n 24); and (iv) leg length (n 7). We also excluded extreme values for age at menarche (before 8 years, n 5; after 18 years of age, n 8)( Reference Midyett, Moore and Jacobson 38 ) and extreme values for the leg length index (index <40·63 cm, n 5; index >53·12 cm, n 8). Thus, our final sample included 12 602 individuals. In addition, the regression models for LBW did not include participants with missing data for this variable (301 men and 348 women).
Response variable
MetS (no, yes) was defined as having at least three of the following components based on the National Cholesterol Education Program Adult Treatment Panel III updated guidelines( Reference Alberti, Eckel and Grudy 1 ): (i) high waist circumference (≥102 cm in men and ≥88 cm in women); (ii) high blood glucose (≥100 mg/dl or use of oral hypoglycaemic drugs or insulin); (iii) low HDL cholesterol (<40 mg/dl for men and <50 mg/dl for women or use of lipid-lowering drugs); (iv) hypertriayclglycerolaemia (TAG ≥150 mg/dl or use of lipid-lowering drugs); and (v) hypertension (blood pressure ≥130/85 mmHg or use of antihypertensive drugs).
All anthropometric and blood pressure measures, as well as the blood samples, were obtained from participants after a 12 h fast. Waist circumference was measured at the mid-point between the lowest rib and the iliac crest( 39 ). Blood pressure was measured three times according to standard procedures with an automated oscillometric sphygmomanometer (Omron 765CP IntelliSense®). The average of the last two measures was used. Biochemical tests were performed according to standard methods: enzymatic colorimetric assay (ADVIA Chemistry) for HDL cholesterol; enzymatic colorimetric method (glycerol phosphate peroxidase; ADVIA Chemistry) for TAG; and enzymatic method with hexokinase (ADVIA Chemistry; Siemens, Deerfield, IL, USA) for blood glucose. All laboratory analyses were performed at a single research centre( Reference Aquino, Barreto and Benseno 34 ).
Explanatory variables
Information on birth weight was obtained by the question ‘According to the information you have, what was your birth weight?’, with the following response options: under 2·5 kg; between 2·5 kg and 4 kg; and over 4 kg. These were subsequently grouped as ≥2·5 kg and <2·5 kg (LBW).
Leg length (in centimetres) was obtained by subtracting sitting height from standing height (in centimetres) obtained according to standardized equipment and techniques using a stadiometer (SECA-SE-216) with a precision of 0·1 cm( Reference Lohman, Roche and Martorell 40 ). Sitting height was obtained with the participants seated on a wooden bench 45 cm high. Relative leg length was obtained using the formula: (leg length/height)×100, and standardized by sex and age. Participants were then classified into three groups: high, mean+1 sd; medium, mean±1 sd; and low, below mean−1sd.
Covariables considered in the analysis were: age (35–44, 45–54, 55–64, 65–74 years); self-reported race/skin colour (white, black or pardo (brown)); education level (university degree, high school, elementary school); physical activity during leisure time (vigorous, ≥3000 MET-min/week; moderate, 600–3000 MET-min/week; light, <600 MET-min/week), where MET=metabolic equivalent of task, measured using the leisure-time domain of the long version of the International Physical Activity Questionnaire( 41 ); smoking (never, former and current smoker); alcohol consumption (no, moderate and excessive), with moderate alcohol consumption defined as <210 g alcohol/week for men and <140 g alcohol/week for women and excessive alcohol consumption defined as ≥210 g alcohol/week for men and ≥140 g alcohol for women; age at menarche in years (continuous), obtained by the question ‘How old were you when you had your first menstrual period?’; and BMI at 20 years old (continuous), obtained through self-reported weight in kilograms at age 20 years divided by the square of current height in metres. BMI was not included due to its strong correlation with waist circumference.
Analyses
All analyses were stratified by gender. We presented the prevalence of MetS according to all the variables in the analysis and statistical associations were examined using Pearson’s χ 2 test with a significance level of 5 %. The χ 2 test for trend was used to assess the trends in the associations when appropriate.
Poisson’s regression with robust variance was used to estimate the magnitude of the association between each explanatory variable (LBW and relative leg length) and MetS. Prevalence ratios (PR) with 95 % CI were presented. After the crude model (Model 0), we added age (in years), self-reported race/skin colour and education level (Model 1); then physical activity, smoking and alcohol consumption (Model 2). For the explanatory variable LBW, we also performed sequential adjustments for relative leg length (Model 3), age at menarche for women only (Model 4) and BMI at 20 years of age (Model 5). For the relative leg length explanatory variable, sequential adjustments were made for LBW (Model 3), age at menarche for women only (Model 4) and BMI at age 20 years (Model 5). The significance level adopted to select variables for inclusion in sequential models was 20 %, and 5 % for the final models. To test for possible heterogeneity in the association of birth weight with MetS according to relative leg length and BMI at 20 years, we added interaction terms to the final model for women only, but the results were not significant. The goodness-of-fit of the final models was assessed by the Hosmer and Lemeshow test.
We conducted a sensitivity analysis excluding participants aged ≥60 years because height loss is related to ageing( Reference Peter, Fromm and Klenk 42 ); this analysis did not reveal any difference in the associations (data not shown).
Analyses were performed using the statistical software package Stata version 12.0.
Results
Of the 12 602 participants included, 40·5 % were between the ages of 45 and 54 years, and most were female, self-reported being white and had a university degree. Almost 74 % of men and 80 % of women engaged in light physical activity during leisure time, 14 % of men and 12 % of women were current smokers, and approximately 13 % of men and 3·5 % of women reported excessive alcohol consumption (Table 1).
Table 1 Percentage distribution of study participants and the prevalence of metabolic syndrome (MetS) according to sociodemographic and health-related behaviours, by gender. Brazilian Longitudinal Study of Adult Health (ELSA-Brasil), 2008–2010
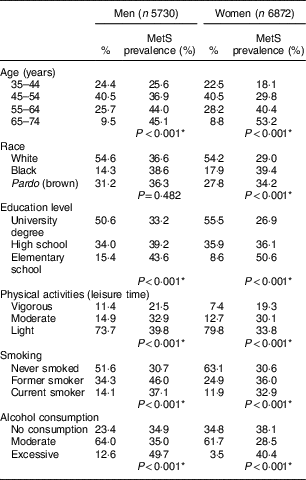
* Pearson’s χ 2 test.
The prevalence of MetS was 34·2 % overall and was higher among men (36·8 %) than among women (32·2 %; prevalence not shown in Table 1). For both sexes, the prevalence of MetS increased with age and decreased with higher levels of education; additionally, MetS was more prevalent among women who self-reported being black. The frequency of MetS was higher for both sexes among those with a lower intensity of physical activity, among former smokers and current smokers, and among those who reported excessive alcohol consumption (Table 1).
Nearly 5·0 % of the participants reported a birth weight of <2·5 kg; additionally, approximately 15·5 % of both men and women had a low relative leg length. Birth weight was associated with MetS among women (P<0·001), but not among men (P=0·178). The lower the relative leg length, the higher the prevalence of MetS for both sexes (Table 2).
Table 2 Percentage distribution of study participants and the prevalence of metabolic syndrome (MetS) according to birth weight and relative leg length, by gender. Brazilian Longitudinal Study of Adult Health (ELSA-Brasil), 2008–2010
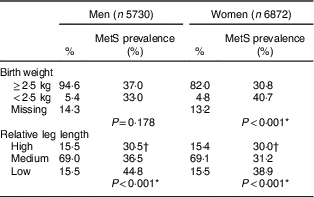
Relative leg length: high, above mean+1 sd; medium, mean±1 sd; low, below mean – 1 sd.
* Pearson’s χ 2 test.
† χ 2 test for trend: P<0·001.
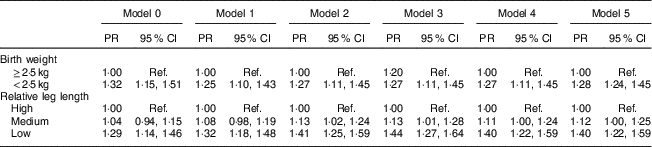
The adjusted models for men are presented in Table 3. Relative leg length was inversely associated with MetS frequency. This association remained significant after adjusting for sociodemographic factors (Model 1), health-related behaviours (Model 2) and BMI at age 20 years (Model 3). We estimated the regression models for LBW and MetS in men but the results were not statistically significant, so they are not presented.
Table 3 Prevalence ratio (PR) and 95 % CI of relative leg length on metabolic syndrome in men. Brazilian Longitudinal Study of Adult Health (ELSA-Brasil), 2008–2010

Ref., reference category.
Relative leg length: high, above mean+1 sd; medium, mean±1 sd; low, below mean−1 sd.
Model 0, crude model; Model 1, Model 0 plus age, race/skin colour and education; Model 2, Model 1 plus physical activities, smoking and alcohol consumption; Model 3, Model 2 plus BMI at age 20 years.
The adjusted models for women are presented in Table 4. The frequency of MetS was 25 % higher (PR=1·25; 95 % CI 1·10, 1·43) among women with a birth weight <2·5 kg than among those with a birth weight ≥2·5 kg after adjusting for sociodemographic characteristics (Model 1), and remained even after further adjustment for health-related behaviours (Model 2), age at menarche (Model 4) and BMI at age 20 years (Model 5).
Table 4 Prevalence ratio (PR) and 95 % CI of birth weight and relative leg length on metabolic syndrome in women. Brazilian Longitudinal Study of Adult Health (ELSA-Brasil), 2008–2010
Ref., reference category.
Relative leg length: high, above mean+1 sd; medium, mean±1 sd; low, below mean–1 sd.
Birth weight: Model 0, crude model; Model 1, Model 0 plus age, race/skin colour and education; Model 2, Model 1 plus physical activities, smoking and alcohol consumption; Model 3, Model 2 plus relative leg length; Model 4, Model 3 plus age at menarche; Model 5, Model 4 plus BMI at age 20 years.
Relative leg length: Model 0, crude model; Model 1, Model 0 plus age, race/skin colour and education; Model 2, Model 1 plus physical activities, smoking and alcohol consumption; Model 3, Model 2 plus birth weight; Model 4, Model 3 plus age at menarche; Model 5, Model 4 plus BMI at age 20 years.
In the crude analysis of female participants, only low leg length in comparison with high leg length was associated with MetS. After adjusting for health behaviours (Model 2), a medium relative leg length was associated with MetS (PR=1·13; 95 % CI 1·02, 1·24) and the strength of the association between low leg length and MetS increased (PR=1·41; 95 % CI 1·25, 1·59). These results remained almost the same after adjusting for birth weight (Model 3), age at menarche (Model 4) and BMI at age 20 years (Model 5), as depicted in Table 4.
The Hosmer and Lemeshow test indicated a good fit of all final models (P>0·05).
Discussion
Our results suggest that intra-uterine and childhood nutritional status may lead to MetS in adult life. LBW and medium and low leg length were independently associated with a higher prevalence of MetS in women, and these associations remained significant even after adjusting for BMI at 20 years old and age at menarche. However, among men, only medium and low relative leg length were associated with MetS.
The direct association between LBW and MetS has been described in previous studies( Reference Li, Jaddoe and Qi 43 – Reference Levy-Marchal and Czernichow 45 ). A meta-analysis showed that LBW newborns had a 2·4-fold greater risk of MetS in adult life( Reference Silveira and Horta 46 ). Although the association between LBW and MetS has previously been reported in men( Reference Barker, Hales and Fall 12 ), we did not identify this association in our study. In a previous study, Dutch women who had been exposed to famine during their first 3 months of gestation, whose mothers were World War II survivors, presented a higher BMI and waist circumference at 50 years than women who had not been exposed to famine during gestation. Notably, these results were not observed among men( Reference Ravelli, Meulen and Osmond 25 ). It is known that LBW increases the risk of death during childhood( Reference Soares, Coutinho and Mascarenhas 47 ). Thus, a sizeable proportion of newborns with LBW may die early in life. This increase in mortality may be more frequent among boys, as they have been reported to have a higher risk of death during the first 4 weeks of life than girls( Reference Gaiva, Fujimori and Sato 48 , Reference Ribeiro, Guimarães and Lima 49 ). These factors may decrease the possibility of identifying a significant association between birth weight and MetS in males.
To our knowledge, the gender difference that we found in the association between LBW and MetS has not been reported previously. Possible explanations for the female-specific relationship between LBW and adult metabolic outcomes might be related to differences in placental development between women and men. It appears that exposure to adverse conditions during pregnancy affects more the placental development of the female fetus than the male fetus( Reference Mandò, Mazzocco and Novielle 50 ). In addition, a recent systematic review showed that in comparison with males, the female placenta increases its permeability to maternal glucocorticoids following maternal stress, a key mechanism linking early development with later-life disease( Reference Carpenter, Grecian and Reynolds 51 ). Because LBW is regarded as a marker of prenatal stress, Carpenter et al. ( Reference Carpenter, Grecian and Reynolds 51 ) argued that females might be more vulnerable to the programming effects of prenatal stress than males.
Despite the hereditary influence, environmental factors are strong determinants of an individual’s final height( Reference Wadsworth, Hardy and Paul 52 ), especially in low- and middle-income countries( Reference Subramanian, Zaltin and Finlay 53 ). Shorter leg length might be a marker of exposure to adverse environmental factors during childhood, particularly malnutrition( Reference Liu, Liu and Li 21 , Reference Whitley, Martin and Smith 54 ), and has been considered the most important component of stature associated with CVD( Reference Ferrie, Langenberg and Shipley 35 ). A recent study showed that greater absolute leg length was negatively associated with MetS in elderly people( Reference Pryzbek and Liu 22 ) and similar results have been observed in children( Reference Liu, Liu and Li 21 ). However, despite much evidence linking shorter leg length and distinct components of MetS, including high systolic blood pressure( Reference Langenberg, Hardy and Breeze 18 ), type 2 diabetes( Reference Liu, Tan and Jeynes 55 , Reference Gunnell, Whitley and Upton 56 ), higher body fat( Reference Frisancho 57 ) and cardiovascular risk( Reference Ferrie, Langenberg and Shipley 35 ), no study so far has investigated the association of leg length with MetS in adults. Moreover, other studies, including one based on the ELSA-Brasil cohort, reported associations of short relative leg length with insulin resistance and type 2 diabetes, independent of birth weight( Reference Mueller, Duncan and Barreto 16 ); these results have also been observed in other studies( Reference Weitzman, Wang and Pankow 17 , Reference Asao, Kao and Baptiste-Roberts 58 ).
The association between medium and low relative leg length and adult MetS observed in the present study did not change after adjusting for LBW in women, suggesting that both are independent markers of adverse exposure during the intra-uterine period and childhood. Thus, our results support the hypothesis that nutritional restriction during pregnancy and childhood has long-term consequences on the genesis of metabolic alterations. They also indicate that the association between malnutrition during childhood and MetS is not influenced by age at menarche among women or by BMI in early adult life, as the strength of the association hardly changed after the adjustments.
Early menarche is often a result of childhood obesity( Reference Salgin, Norris and Prentice 59 ) and is associated with increased risk of obesity( Reference Prentice and Viner 60 ), MetS( Reference Akter, Jesmin and Islam 61 , Reference Stöckl, Meisinger and Peters 62 ), CVD( Reference Prentice and Viner 60 , Reference Dreyfus, Jacobs and Mueller 63 ) and diabetes( Reference Mueller, Duncan and Barreto 31 , Reference Conway, Shu and Zhang 64 ) in adult life. Additionally, early menarche is associated with shorter leg length in different populations( Reference Onland-Moret, Peeters and Gils 30 , Reference Schooling, Jiang and Lam 65 ), and a high level of oestrogen at the beginning of puberty is a determinant of cessation in the linear growth of long bones and thus of the legs( Reference Salgin, Norris and Prentice 59 , Reference Conway, Shu and Zhang 64 ). In the present study, low leg length remained associated with MetS, even after adjusting for age at menarche, with no alterations in the strength of the association. Therefore, our results do not suggest that age at menarche has a relevant role in the development of MetS.
The strengths of our study are the size of the population and the methodological rigour( Reference Schmidt, Duncan and Mill 32 ). Height has increased in younger populations, and this cohort effect makes it difficult to study measures such as leg length without accounting for this effect. In the present work, however, leg length measures were standardized by sex and age, and it is thus very unlikely that a cohort effect remained in the association between leg length and MetS.
Although the present study was cross-sectional, it is improbable that the associations between markers of malnutrition in childhood and MetS were due to reverse causality because they preceded the analysed outcome. The associations are likely underestimated because cross-sectional studies are composed of survivors and individuals exposed to more severe malnutrition during childhood may have a lower survival rate due to MetS-related events. As the ELSA-Brasil population does not represent the entire Brazilian population, the estimated prevalence of MetS and of adverse markers of child nutrition cannot be generalized to the general population; however, it is unlikely that this limitation decreases the internal validity of the associations found.
Birth weight was self-reported and it is possible that men provided less accurate information than women, leading to a non-differential misclassification of male participant data and thus decreasing the possibility of identifying a significant association between birth weight and MetS in males. Body weight at 20 years old and age at menarche are also prone to recall bias. Although not probable, we cannot discount the possibility that compared with those without abdominal obesity, people with abdominal obesity (who were thus more likely to have MetS) more frequently reported a lower weight than they actually had when they were young. However, recall bias for age at menarche is much less probable because this event is a very important experience for teenagers; furthermore, a study showed that real and reported age at menarche did not differ after 33 years( Reference Must, Phillips and Naumova 66 ). Although there were missing data on birth weight, they might have been missing at random and probably not have a significant effect on the conclusions.
Our results support the hypothesis that early-life nutritional conditions as estimated by LBW and lower leg length may contribute to the development of MetS in the studied population. In addition, our study results indicate that markers of adverse exposures in utero and during childhood, such as LBW and low relative leg length, may contribute to metabolic alterations in adulthood. The lack of a significant association between LBW and MetS in men, however, deserves further investigation. The present study contributes to the literature on the burden of non-communicable diseases associated with poor nutrition in early life, especially in middle- and low-income countries where exposures to such adverse conditions are more prevalent. New research areas, primarily focusing on incident MetS, shall contribute to a better understanding of these associations and to the design of interventions aimed at preventing adverse outcomes in early phases of life.
Acknowledgements
Acknowledgements: The authors thank the staff and participants of the ELSA-Brasil for their important contributions. Financial support: This work was supported by the Brazilian Ministry of Health (Department of Science and Technology) and the Ministry of Science, Technology and Innovation (Financiadora de Estudos e Projetos (FINEP) and National Research Council (CNPq) grant numbers 01 06 0010.00, 01 06 0212.00, 01 06 0300.00, 01 06 0278.00, 01 06 0115.00 and 01 06 0071.00). B.L.B. was supported by a master degree research fellowship of the Universidade Federal de Ouro Preto. L.G., S.M.B., M.I.S., M.C.B.M., S.M.A.M. and G.V.-M. are research fellows of the CNPq (Brasilia, Brazil). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Conflict of interest: None. Authorship: B.L.B., L.G., J.F.A. and S.M.B. contributed to study conception, analysis and interpretation of data, manuscript drafting and critical manuscript revision for important intellectual content. M.F.H.S.D., M.C.B.M., S.M.A.M., L.O.C., G.V.-M. and M.I.S. contributed to critical manuscript revision for important intellectual content. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Committee of Ethics in Research (approval number 189/2006). Written informed consent was obtained from all subjects.









