Although early childhood development is known to be key in determining the future health and education of children(Reference Feinstein and Duckworth1), inadequate developmental achievement has persisted in resource-constraint settings despite available interventions(Reference Engle, Fernald and Alderman2). In line with this, 250 million children below 5 years in developing countries are at risk of not reaching their full developmental potential(Reference Black, Walker and Fernald3). This is largely due to poverty, undernutrition, poor health and unstimulating living environments(Reference Grantham-McGregor, Cheung and Cueto4). Stunting (linear growth restriction) is often considered as a marker of chronic undernutrition and may impact negatively on cognitive development(Reference Grantham-McGregor, Cheung and Cueto4). In Uganda, 29 %, 11 % and 4 % of the children below 5 years are stunted, underweight and wasted, respectively(5). Early childhood development promotion in Uganda has previously been found to be inadequate: Over 75 % of children were found not to receive psychosocial stimulation like toys and learning activities, e.g. counting, at an early age(Reference Britto, Engle and Alderman6).
The first years of life are fundamentally crucial for brain development and functioning(Reference Bruder7,Reference Block, Dreyer and Cohen8) . During this period, the brain grows up to about 80 % of its adult weight, and any nutritional deficiencies can cause significant and permanent damage(Reference Bryan, Osendarp and Hughes9,Reference Lenroot and Giedd10) . Therefore, identifying and treating developmental issues at an early stage could prevent disability and improve long-term health outcomes like physical, social and emotional well-being(Reference Siller, Morgan and Turner-Brown11–Reference Chaudhari and Kadam13). The Lancet series on early childhood development highlighted the consequences of poor child development due to poverty and stunting. The series emphasised the need to promote child development during the critical window of opportunity (i.e. the first 1000 d of life) in order to prevent short- and long-term health effects like disability and death and to minimise the impact on individual incomes and countries’ gross national product(Reference Black, Walker and Fernald3,Reference Richter, Daelmans and Lombardi14,Reference Britto, Lye and Proulx15) .
Dietary diversity score (DDS) is the number of food groups consumed over a reference period(Reference Ruel16). It is a useful indicator of dietary quality, nutrient adequacy and nutritional status of children(Reference Ruel17). The WHO defines the minimum dietary diversity as the proportion of children 6–23 months of age who receive foods from four food groups or more(18). Consumption of four or more food groups was found to be associated with better quality diets for children(19) and this would imply that on top of consuming a staple food, the child was more likely to consume at least one animal-based food and at least one fruit or vegetable that day and thereby achieve micronutrient adequacy(20). Timely introduction of foods (solid, semi-solid or soft foods) at 6 months of age alongside breast-feeding is important to fill the nutritional gaps left by breast milk(18,Reference Imdad, Yakoob and Bhutta21) . It is during this early period that the incidence of stunting is highest because children have high demand for nutrients and often the quality and quantity of food available are limited(Reference Dewey and Adu-Afarwuah22,Reference Shrimpton, Victora and de Onis23) . This period has previously been highlighted as critical for promotion of growth and development especially in developing countries where often inappropriate child feeding results in growth faltering(Reference Imdad, Yakoob and Bhutta21,Reference Shrimpton, Victora and de Onis23,Reference Victora, de Onis and Hallal24) .
Although studies have examined associations between nutritional status and specific nutrients with early childhood development(Reference Black, Walker and Fernald3,Reference Richter, Daelmans and Lombardi14,Reference Britto, Lye and Proulx15,Reference Georgieff25) , the broader diversity of children’s diets in relation to development competencies is one area that has received less attention. This makes it important to study the diversity of children’s diets as a whole in relation to child development. Timely initiation of diverse child feeding is also thought to be important, but has been little explored.
Understanding how early childhood diet relates to developmental outcomes will support the design and implementation of future interventions. To our knowledge, no study has examined the association between dietary diversity at 6–8 months of age and child development at 20–24 months in rural Africa.
Between October 2013 and August 2014, a nutritional education intervention, the ‘Child Nutrition and Development’ (CHNUDEV) study was conducted in Kabale and Kisoro districts of south-western Uganda (https://www.med.uio.no/imb/english/research/projects/chnudev-study/). To investigate whether early childhood dietary diversity was associated with child development, we conducted a secondary analysis of data from this study to examine the relationship between child dietary diversity at 6–8 months (baseline) and five child development domains (communication, gross motor, fine motor, personal–social and problem solving) measured by the parent-reported Ages and Stages Questionnaire (ASQ) at 20–24 months (end line). We also examined if other child- and maternal-related factors at baseline could predict child development outcomes at 20–24 months.
Materials and methods
Study design
The study was an exploratory secondary analysis of data from a cluster-randomised controlled trial that included 511 mother–child pairs. The trial was a longitudinal study with data collected at three time points; the first was at enrolment (baseline) when children were at 6 to 8 months of age, then midline at 12–16 months and the final one at 20 to 24 months of age. A total of ten subcounties participated in the original study of which five were randomly allocated to the intervention and the other five to the control arm. The intervention was nutrition, sanitation and stimulation education delivered to groups of mothers/caretakers by trained persons. Behavioural change communication technique was used to deliver messages on the guiding principles of complementary feeding, good hygiene practices, child stimulation and food preparation. Each group of mothers had a leader who in most cases would be a member of the village health team. The team leader was responsible for following up the group members and encouraging them to adhere to the intervention. The intervention was delivered for 6 months. For the current study, data from two time points were used, with dietary diversity and other child and household characteristics taken from baseline at 6–8 months of age and outcome variables (child development domains) assessed at 20–24 months of age. More details of the trial and the sampling procedures have previously been described(Reference Muhoozi, Atukunda and Diep26).
Study setting and study population
The data used in this study were collected at two time points from two districts (Kabale and Kisoro) in the south-western region of Uganda. This hilly region of the country is predominantly occupied by subsistence farmers who cultivate small pieces of land. In order to reduce differences in socio-economic status, the study excluded town centres. This region was chosen because of the high rate (33 %) of under five stunting that was far above the national figure(27). The study population were children below 24 months of age.
Sample size
With a sample of 390 available participants considered for this analysis, we used the Kelsey and colleagues formula to estimate how much power the sample gives us to assess the desired associations(Reference Kelsey, Whittemore, Evans and Kelsey28). Based on literature, the least expected proportion of children with normal development at 24 months for any of the five development domains (communication, gross motor, fine motor, personal–social and problem solving) was reported for communication skills as 74·8 %(Reference Muhoozi, Atukunda and Mwadime29). Assuming a 15 % incremental change in this proportion, a standard normal value corresponding to the 95 % CI and a 5 % margin of error, our sample of 390 participants gave us a power of 80 % which was sufficient. However, only 385 children had complete data on key variables and therefore were used in this analysis.
Data collection and assessment tools
Child development was assessed using the ASQ third edition(Reference Squires, Bricker and Twombly30), a parent/caregiver completed screening tool(Reference Kerstjens, Bos and ten Vergert31) used to report a wide range of adaptive behaviours, and previously used in similar settings(Reference Hornman, Kerstjens and de Winter32,Reference Small, Hix-Small and Vargas-Baron33) . In order to minimise interruptions, assessment was performed in hired special rooms. A mobile tent was used in cases where rooms were not available. All mothers/caregivers responded to the questions and provided parental reports. For mothers/caregivers who could not read the translated ASQ tool in the local language, the assessments were conducted together with the data collection team. This team would read the ASQ questions to the mothers and then they would score the results together. Notably, five women (1·3 %) could not read the local language. The ASQ is designed to identify young children with delays in development and those that need further evaluation. The tool is made up of twenty-one development intervals, each consisting of thirty items in five domains of developmental assessment including communication, personal–social, problem solving, gross motor and fine motor(Reference Squires, Bricker and Twombly30,Reference Squires, Bricker and Potter34,Reference Bricker, Squires and Mounts35) . The thirty items (six items for each domain) in the translated ASQ resulted into a satisfactory internal reliability to test each of the childhood development (Cronbach’s α: communication = 0·910; gross motor = 0·870; fine motor = 0·789; problem solving = 0·730; personal–social = 0·758). For each of the domains, the scores were calculated on a scale of 0 to 60 points (worst to best). The child development domains scores were then categorised into groups in accordance with the ASQ tool cut-offs: normal, delayed and needs attention. In this analysis, the child development domains (outcome variable) were regrouped into two categories: normal and delayed/needs attention. The cut-off (normal and delayed/needs attention) points include 36 points for gross motor, 36·4 points for fine motor, 36·5 points for communication, 32·9 points for problem solving and 35·6 points for personal–social(Reference Gollenberg, Lynch and Jackson36).
Data on household characteristics and child dietary data were collected at baseline using a questionnaire consisting of both open- and close-ended questions. The questionnaire was administered to the child’s primary caregiver through an interview. Dietary diversity was scored on a scale of 0 to 8 food groups. The scores were adapted from the household DDS tool which has been previously validated for use in developing countries(Reference Swindale and Bilinsky37). This tool consists of eight food groups including (i) grains, roots or tubers; (ii) vitamin A-rich plant foods; (iii) other fruits or vegetables; (iv) meat, poultry, fish, seafood; (v) eggs; (vi) pulses/legumes/nuts; (vii) milk and milk products and (viii) foods cooked in oil/fat. Notably, breast milk is not one of the food groups assessed by this tool. Any of these food groups consumed by the child in the past 24 h was given a score of one, and the scores were added up to obtain the child DDS.
The Uganda poverty score card(Reference Schreiner38) was used to obtain poverty scores. The scores were then added and compared with the poverty likelihood on a scale of 0–100 (least to most likely to be below the poverty line) with a score of 70·8 and above being considered extreme poverty. For the purpose of this analysis, poverty likelihood data were received as a three-category variable constructed during statistical analysis of the original trial. The three categories were extreme poverty, moderate poverty and well off.
In the original study, child morbidity was assessed by asking the mothers/caretakers. Any illness at the time of the study or in the previous 2 weeks prior to the study was assessed. The most common reported illnesses were diarrhoea, cough, common cold and fevers. The current study considered only illness status at the time of data collection.
To increase reliability of the tools, the ASQ and the socio-demographic questionnaires were originally pretested on children of the same age group in a similar setting before the actual assessment. The interviewers were trained on how to administer the study tools in advance so as to reduce inter-observer bias.
Statistical analysis
Data were analysed using Stata version 15.0. Baseline characteristics of the participants were tabulated as frequencies and percentages for categorical variables and means/median and sds/interquartile range for continuous variables. To assess for the association between dietary diversity and other baseline factors at 6–8 months and childhood development at 20–24 months, we conducted bivariate and multivariate analyses. For bivariate analysis, we explored the association between each predictor and outcome to obtain crude ORs at the 95 % CI using multilevel mixed effects logistic regression(Reference Dayton39,Reference Peng, Lee and Ingersoll40) , adjusting for clustering at sub-county level. In all the multilevel analyses, sub-counties provided the level two random intercepts.
For each child development domain (outcome), a multivariable logistic regression model was built to establish its association with dietary diversity and other baseline variables, reporting results as adjusted ORs and their corresponding 95 % CIs. The multivariable models were built using a mixed approach of variable selection(Reference Heinze, Wallisch and Dunkler41). All covariates with a P-value of < 0·1 at bivariate level of analysis were considered candidates for the multivariable models. We then employed logical model building approach following the conceptualisation of the study outcome, literature and theoretical framework to select the final variables to include in the multivariable models(Reference Bursac, Gauss and Williams42). Some variables like child age, child sex and poverty likelihood were considered and included in all models as a priori confounders(Reference Brookhart, Stürmer and Glynn43). Maternal/caregiver nutritional education was the intervention tested in the original trial and we included it in the models for the current analysis to adjust for the intervention effect. All covariates were tested for collinearity using the variance inflation factor. In this case, mother’s number of biological children and child birth order were found to be collinear, hence child birth order was omitted from the final models. The primary exposure (DDS) was included in the model as a continuous variable after passing linearity assumptions with the outcome variables. We used the White’s test to test for homoscedasticity and the augmented component plus residual plot to test for the linearity between DDS and the five developmental domains (see online supplemental file 1). For each model, covariates with a P-value of < 0·05 after multivariable analysis were considered statistically significant.
Results
Baseline characteristics
Of the 385 children who were analysed, 200 (52 %) were boys and 185 (48 %) were girls.
The mean DDS of children at 6–8 months was 2·9 food groups. Most of the households were living in moderate to extreme poverty with only 14 % classified as being well off at the time of baseline data collection. A third (33 %) of the children were ill at the time of data collection at 6–8 months age. Other baseline characteristics are presented in Table 1, and a breakdown of these characteristics by development domains is attached in supplemental file 2 (Table 1). The distribution of developmental domains at baseline is also presented in supplemental file 2 (Table 2).
Table 1 Baseline characteristics (n 385)

IQR, Interquartile range.
Table 2 Association of dietary diversity and other factors with child development outcomes – bivariate analyses
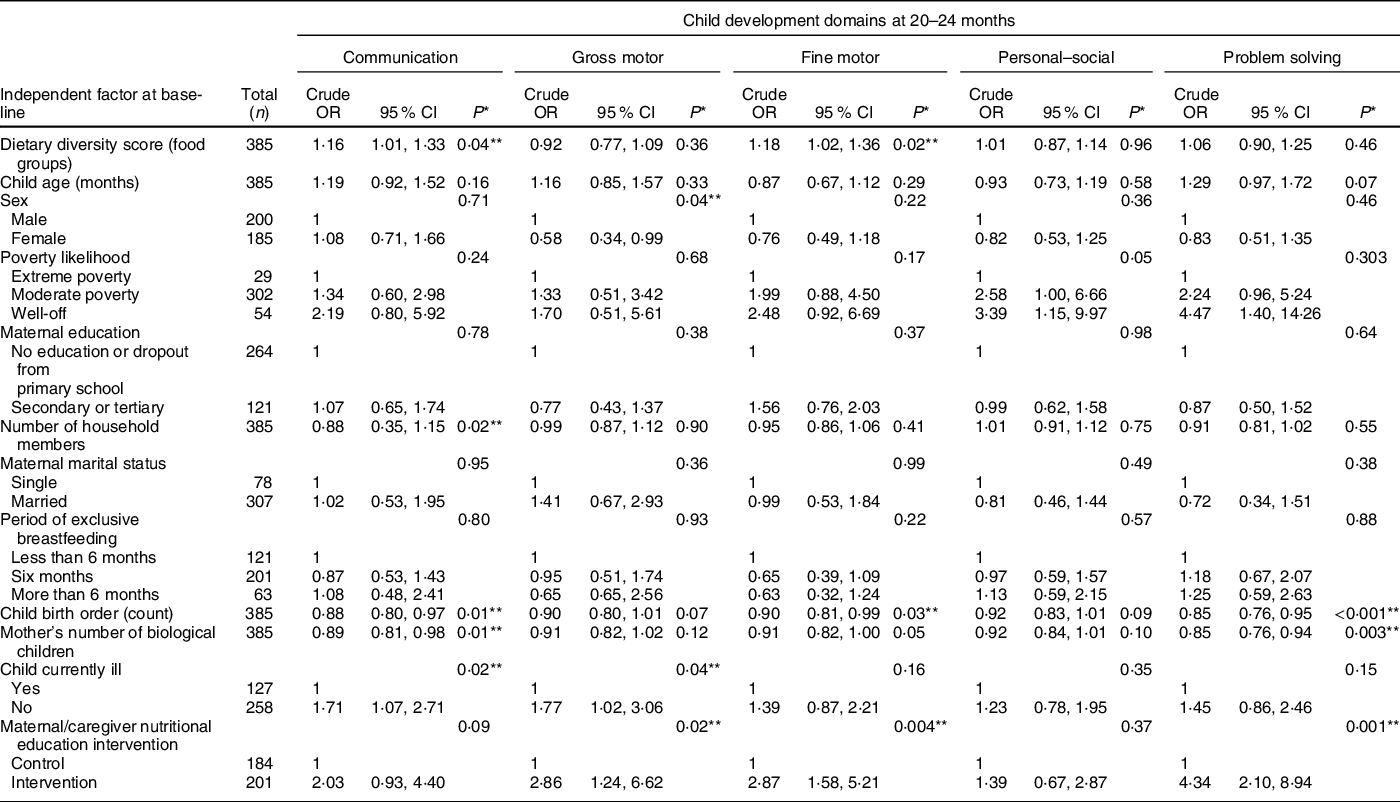
* P-values were obtained via likelihood ratio test.
** Significant P value.
Association between dietary diversity and other baseline characteristics with the child development outcomes at 20–24 months – bivariate analyses
The bivariate analysis in Table 2 shows that the child DDS at 6–8 months (baseline) was positively associated with normal communication and fine motor skills at 20–24 months, but not with gross motor, personal–social and problem-solving skills.
We next performed similar bivariate analyses of other baseline factors we presumed could be important for child development at 20–24 months. Notably, we found significant associations between communication skills and child illness status, mother’s number of biological children, child birth order and household size. Development of gross motor skills was significantly associated with sex, maternal/caregiver nutritional education intervention and child illness status. Furthermore, child birth order and nutritional education of the mothers/caregivers were significantly associated with development of fine motor skills. In addition, development of problem-solving skills was significantly associated with maternal/caregiver nutritional education intervention, child birth order and mother’s number of biological children. We did not find any factor associated with personal–social development (P > 0·05), except that poverty likelihood was borderline associated with personal–social abilities (P = 0·05).
Association between dietary diversity and other baseline characteristics with the child development outcomes at 20–24 months – multivariate analyses
After adjustments for possible confounders, we found a significant association between DDSs at baseline and the development of fine motor skills, so that for every additional food group in the child’s diet at baseline there was 18 % higher odds of having normal fine motor skills at 20–24 months (OR = 1·18; CI 1·01, 1·37; P = 0·02). We found no significant associations between baseline DDSs and communication, gross motor, personal–social or problem-solving skills at 20–24 months (Table 3).
Table 3 Association of dietary diversity and other factors with child development outcomes – multivariate analyses
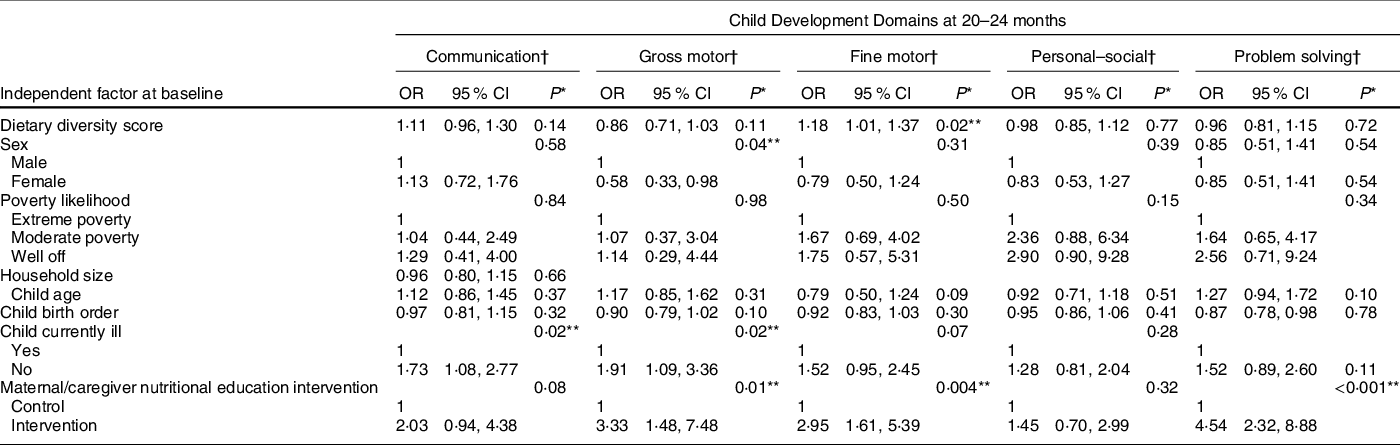
* P-values were obtained via likelihood ratio test.
† Models for all the development domains were adjusted for dietary diversity scores at 6–8 months, sex, poverty likelihood, child age at baseline, child birth order, child illness and nutritional education intervention (trial assignment).
** Significant P value.
Finally, we performed multivariate analyses of possible associations between other baseline factors and child development outcomes at 20–24 months (Table 3). Absence of child illness was significantly associated with having normal communication skills, so that relative to children who were sick, those who were not sick had 73 % higher odds of developing normal communication skills. Maternal/caregiver nutritional education intervention, child illness status and sex were significantly associated with development of gross motor skills.
Children whose mothers/caretakers received nutritional education intervention were nearly three times more likely to have normal development of fine motor skills compared with those who did not. There was also a significant association between maternal/caregiver nutritional education intervention and development of problem-solving skills, so that children whose mothers/caregivers received nutritional education intervention were 4·54 times more likely to have normal development of problem-solving skills compared with those whose mothers/caregivers did not receive nutritional education. No factors were significantly associated with development of child personal–social skills.
Discussion
The main aim of this secondary data analysis of our cluster-randomised controlled trial (26) was to examine possible associations between dietary diversity at start of the randomised controlled trial when the children were 6–8 months (baseline) and child development across the five developmental domains (at 20–24 months). We also analysed possible association of other independent factors at baseline and the development outcomes at 20–24 months. The multivariate analyses showed that child dietary diversity at 6–8 months of age was significantly associated with improved fine motor development at 20–24 months of age. No significant associations were found between child dietary diversity and the other development domains. In addition, absence of illness at 6–8 months was associated with development of communication skills at 20–24 months. Moreover, development of gross motor skills at 20–24 months was predicted by maternal/caregiver nutritional education intervention, absence of child illness and child sex. Finally, maternal/caregiver nutritional education intervention was also significantly associated with development of fine motor and problem-solving skills.
There are few reports directly relating early child dietary diversity and later development, as assessed in this study. Those that did so considered an aggregated ASQ score (as a continuous variable) for all the development domains (communication, fine motor, gross motor, personal–social and problem solving)(Reference Thorne-Lyman, Shrestha and Fawzi44,Reference Miller, Neupane and Joshi45) . Our study, however, elaborates on how dietary diversity influences each of the domains separately; as the ASQ does not provide a uniform cut-off point to categorise children as normal, delayed and needs attention when an aggregated score for all domains is used, but this is provided for when individual domains are considered.
In Nepal, dietary diversity in the early life of a child was associated with development(Reference Thorne-Lyman, Shrestha and Fawzi44,Reference Miller, Neupane and Joshi45) . A study among Guatemalan children showed that a diet high in protein was positively associated with early motor development(Reference Kuklina, Ramakrishnan and Stein46) although this study assessed general motor development and not specifically fine motor. A cross-sectional study in India identified dietary diversity to be associated with child development(Reference Larson, Young and Ramakrishnan47). A diet high in nutritious foods among Guatemalan children 0–36 months of age also improved later educational performance(Reference Maluccio, Hoddinott and Behrman48).
The association between dietary diversity and fine motor development could be explained by the fact that dietary diversity is a good proxy for micronutrient intake,(18) and different micronutrients have been shown to improve brain function. For example, studies on Fe(Reference Grantham-McGregor and Ani49), Zn(Reference Shah and Sachdev50), iodine(Reference Melse-Boonstra and Jaiswal51–Reference Zimmermann53), folic acid and vitamin B12 (Reference Dror and Allen54,Reference Zhang, Huang and Tian55) have demonstrated associations between these micronutrients and brain development.
Undernutrition could be a mediating factor between dietary diversity and motor development. A meta-analysis of studies in low- and middle-income countries found stunting to be associated with poor motor development(Reference Sudfeld, McCoy and Danaei56). It is also possible that dietary diversity could have an influence on the amount of stimulation that the child gets in a number of ways. First, it is well known that the child’s and mother’s diet are correlated(Reference Leroy, Olney and Ruel57) and that the mother is usually the main source of child stimulation. Second, it is also possible that children who had higher DDSs were born to mothers or were being taken care of by people who not only had time for feeding them but also playing and stimulating them. Indeed, a study in Bangladesh indicated that mothers who feed their children well are likely to provide more stimulation as well(Reference Aboud and Akhter58). A more diverse diet could also mean good quality nutrients to improve bone health and physical strength hence better motor performance as evidenced from previous studies in Kenya(Reference Neumann, Jiang and Weiss59,Reference Neumann, Murphy and Gewa60) .
Another interesting finding was the significant association between child illness status with communication and gross motor skills development. Children aged 6–8 months who were found ill were less likely to have normal development of the two domains in this study. This may be because sickness reduces the ability of children to play with others and this may hinder their development. Sickness could also affect the functioning of their limbs and hence inadequate motor development as similar results were reported in the first of the three Lancet series on child development. The series highlighted the fact that illness and poor health are among the factors that delay child development in developing countries(Reference Black, Walker and Fernald3). Evidence from animal models has previously shown that infections in early life increase the risk of central nervous system disorders(Reference Boksa61). Further, children who are ill are more likely to have reduced intake and utilisation of food, hence prone to malnutrition which in turn can affect their development(Reference Guerrant, Oriá and Moore62,Reference Berkman, Lescano and Gilman63) . Therefore, prevention and early treatment of childhood illnesses, especially in resource-constrained settings, may be paramount for optimum early childhood development.
The finding that girls were less likely to develop normal gross motor skills than boys is in line with studies that have indicated that boys were better off than girls in terms of development of specific aspects of the brain(Reference De Bellis, Keshavan and Beers64,Reference Ingalhalikar, Smith and Parker65) . Culturally, in Uganda, parents were found to encourage boys to play more and be dominant later in life(Reference Mirembe and Davies66). On the contrary, recent studies showed that girls had a biological advantage in terms of brain development abilities compared with boys(Reference Hampson67,Reference Haapala, Eloranta and Venäläinen68) .
As found in the original trial analysis(Reference Muhoozi, Atukunda and Diep26), nutritional education intervention delivered to mothers/caregivers from when the children were 6–8 months up to 12–16 months promoted gross motor, fine motor and problem-solving skills development at 20–24 months. These results are also consistent with a systematic review by Grantham McGregor and colleagues in which nutritional interventions were not only beneficial for improving nutritional status but also child development(Reference Grantham-McGregor, Fernald and Kagawa69). Therefore, behavioural change communication messages regarding nutrition delivered to rural mothers/care takers may be an effective and sustainable way of promoting child development.
This study had a number of strengths: The participants were recruited from a randomised controlled trial. The ASQ is widely used and has been validated(Reference Gollenberg, Lynch and Jackson36,Reference Juneja, Mohanty and Jain70) . Further, our study examined the broader aspect and timely initiation of dietary diversity in relation to child development. This is an area that has received less attention in the past and to the best of our knowledge, we present current knowledge which has not been assessed previously in the African setting. Lastly, our study incorporated and assessed risk factors for a number of childhood developmental outcomes rather than a single developmental domain, as many previous studies have done. This makes it very relevant to understand childhood development holistically and address it appropriately because in most cases as observed in this study, risk factors for different developmental parameters tend to overlap.
Our study had some limitations: While we adjusted for key child and maternal factors, we did not have adequate proxies for some key factors like mother–child interaction and stimulation, the nature of home environment or general social support. Some would argue that estimating risk ratios could have been more appropriate for our analyses because ORs tend to overestimate the strength of association, especially when the prevalence of the outcome is more than 15 %. However, we used ORs because they are easier to interpret with regard to our study. Further, we did not have quantitative information about the actual food intakes. Our study could have been slightly underpowered as the sample size was less by five participants who were dropped due to incomplete data on ASQ developmental domains. However, we acknowledge that this is one of the key challenges of using secondary data(Reference Cheng and Phillips71). Although we pre-tested the ASQ, the tool thresholds are from a high-income country which may not adequately represent a low-income population. Whereas different illnesses could impact the development domains differently, information on specific illnesses among the children was not collected in the original trial. Finally, although our findings could be generalised to Uganda and the East-African region, the results may not be generalisable to the rest of the world due to variations, e.g. in diet.
Conclusion
In conclusion, we found that child DDSs at 6–8 months of age were significantly associated with improved fine motor skills development at 20–24 months of age. In addition, absence of illness at 6–8 months was significantly associated with the development of communication skills at 20–24 months, whereas development of gross motor skills was predicted by maternal/caretaker nutritional education intervention, absence of child illness and child sex. Finally, maternal/caregiver nutritional education intervention was also significantly associated with the development of fine motor and problem-solving skills.
Acknowledgements
Acknowledgements: The authors would like to thank the participating communities and the participants for accepting to take part in the original trial. Financial support: The original trial was funded by Throne Holst Foundation and the University of Oslo. P.K. also received funding from Commonwealth Scholarship Commission as part of his scholarly project. Conflicts of interest: There are no conflicts of interest. Authorship: A.C.W., P.O.I. and G.M. designed and supervised the trial; P.K., C.M. and G.M. developed the study for the current paper; P.K., G.M. and P.A. collected the data; P.K., C.M. and N.M. analysed the data. All the authors participated in the manuscript preparation and approved the final draft for submission. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving research study participants were approved by the London School of Hygiene and Tropical Medicine Ethics Committee. The original trial was approved by the Uganda National Council for Science and Technology and the Norwegian Regional Committee for Medical and Health Research Ethics and is registered at ClinicalTrials.gov as NCT02098031. Written informed consent was obtained from all subjects in the original trial.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S136898002100077X







