Introduction
Pleasure and rewards constitute vital attractors that not only shape our momentary decisions, but also motivate future behavior. Deficits in reward processing have been proposed as a candidate mechanism underlying anhedonia, which describes a consistently diminished pleasure and interest in almost all daily life activities (Halahakoon et al., Reference Halahakoon, Kieslich, O'Driscoll, Nair, Lewis and Roiser2020), (research domain criteria framework: positive valence system) (Insel et al., Reference Insel, Cuthbert, Garvey, Heinssen, Pine, Quinn and Wang2010). Anhedonia is a cardinal symptom of major depressive disorder (MDD) and it represents a transdiagnostic symptom of several debilitating neurological and mental health disorders (Husain & Roiser, Reference Husain and Roiser2018). With currently over 320 million people living with depression, MDD has become a leading cause of disability worldwide (Friedrich, Reference Friedrich2017). While the established interventions can alleviate some of the symptoms of depression, anhedonia is not only inefficiently targeted by the available treatments, but it is also highly treatment resistant (Becker, Reference Becker2022; Kendler, Reference Kendler2016; Robbins, Reference Robbins2016; Treadway & Zald, Reference Treadway and Zald2011). Understanding the specific dysfunctions of behavioral and neural reward processing in depression may thus provide better treatment and management options (Oh, Lee, Patriquin, Oldham, & Salas, Reference Oh, Lee, Patriquin, Oldham and Salas2021).
Reward processing encompasses an entire array of subprocesses that can be defined based on different perspectives including the class of external reinforcers as well as the stage and function of the rewarding process. Several stimuli can serve as rewarding reinforcers in humans, including money, palatable food, and positive social interactions. These rewards share behavioral and neural features, such as motivational properties and engagement of the dopaminergic mesocorticolimbic pathways (Sescousse, Caldú, Segura, & Dreher, Reference Sescousse, Caldú, Segura and Dreher2013), including activation of the ventral striatum (Mas-Herrero, Maini, Sescousse, & Zatorre, Reference Mas-Herrero, Maini, Sescousse and Zatorre2021). Monetary rewards are the most commonly used experimental paradigms in basic and psychiatric human neuroscience, but in addition to shared neural pathways (Ait Oumeziane, Schryer-Praga, & Foti, Reference Ait Oumeziane, Schryer-Praga and Foti2017; Flores, Münte, & Doñamayor, Reference Flores, Münte and Doñamayor2015), differential neural responses and alterations in patients with depression have been reported for monetary and naturalistic stimuli such as food and social rewards (Ait Oumeziane, Jones, & Foti, Reference Ait Oumeziane, Jones and Foti2019; Rademacher et al., Reference Rademacher, Krach, Kohls, Irmak, Gruender and Spreckelmeyer2010; Spreckelmeyer et al., Reference Spreckelmeyer, Krach, Kohls, Rademacher, Irmak, Konrad and Gründer2009; Yang et al., Reference Yang, Liu, Zeng, Wu, Zhao, Xin and Becker2021).
Most fMRI incentive delay tasks employ monetary and social rewards that are generally considered to be secondary and learned, and can be differentiated from food as a primary reward. Monetary rewards differ from all other rewards in important aspects. In particular, (a) they possess instrumental or exchangeable value and hence, can be used to acquire other desired goals, and (b) the receipt of monetary rewards commonly has a stronger association with the performance or efforts of the participant at least in the prevailing experimental paradigms (Sescousse et al., Reference Sescousse, Caldú, Segura and Dreher2013). In contrast, natural rewards (often) do not possess an instrumental value and often have a less direct association with performance or effort. Although both reward types are strong motivational drivers in humans, natural rewards prevail in daily life and the pursuit of these rewards could be increased via therapeutic interventions in depression. The prevailing focus on monetary rewards in experimental studies may limit the generalizability and translation of altered reward-related brain processes in mental disorders, see (Ait Oumeziane et al., Reference Ait Oumeziane, Jones and Foti2019) or (Zimmermann et al., Reference Zimmermann, Kendrick, Scheele, Dau, Banger, Maier and Becker2019). Within this context, we focused on the comparison of monetary v. natural reward (encompassing palatable food and social rewards) processing alterations in depression.
On the neural level, reward processing has been closely linked to activation in mesocorticolimbic systems, including the striatum and amygdala as well as medial prefrontal and anterior cingulate regions (Rizvi, Pizzagalli, Sproule, & Kennedy, Reference Rizvi, Pizzagalli, Sproule and Kennedy2016; Schultz, Reference Schultz2015). While some evidence suggests that the specific neural systems vary as a function of the reinforcers and the stage of the reward process (Martins et al., Reference Martins, Rademacher, Gabay, Taylor, Richey, Smith and Paloyelis2021; Oldham et al., Reference Oldham, Murawski, Fornito, Youssef, Yücel and Lorenzetti2018; Yang et al., Reference Yang, Liu, Zeng, Wu, Zhao, Xin and Becker2021), the ventral striatum has been consistently engaged during anticipation and receipt of monetary rewards (Jauhar et al., Reference Jauhar, Fortea, Solanes, Albajes-Eizagirre, McKenna and Radua2021), as well as the receipt of different natural rewards. According to overarching frameworks (Fehr & Camerer, Reference Fehr and Camerer2007; Izuma, Reference Izuma and Toga2015), these meta-analytic findings may support the notion of a ‘common neural currency’ for reward. However, (Knutson & Bossaerts, Reference Knutson and Bossaerts2007) suggests phylogenetically different classes of rewards.
Previous meta-analyses have provided convergent evidence for reward processing deficits in depression (Halahakoon et al., Reference Halahakoon, Kieslich, O'Driscoll, Nair, Lewis and Roiser2020), with functional neuroimaging studies suggesting altered neural activity during reward processing (Keren et al., Reference Keren, O'Callaghan, Vidal-Ribas, Buzzell, Brotman, Leibenluft and Stringaris2018; Yang et al., Reference Yang, Su, Yang, Song, Yan, Luo and Zeng2022). Alterations have been observed along the mesocorticolimbic reward circuit, including prefrontal regions such as the orbitofrontal cortex (OFC), medial prefrontal cortex (mPFC) and the anterior cingulate cortex (ACC), as well as subcortical regions including the striatum, amygdala, and hippocampus (Geugies et al., Reference Geugies, Mocking, Figueroa, Groot, Marsman, Servaas and Ruhé2019; Keren et al., Reference Keren, O'Callaghan, Vidal-Ribas, Buzzell, Brotman, Leibenluft and Stringaris2018; Ng, Alloy, & Smith, Reference Ng, Alloy and Smith2019; Russo & Nestler, Reference Russo and Nestler2013). Neurofunctional alterations in the striatum and ACC during monetary reward processing have been consistently observed during different stages of depression, including subthreshold depression (Stringaris et al., Reference Stringaris, Vidal-Ribas Belil, Artiges, Lemaitre, Gollier-Briant, Wolke and Paillère-Martinot2015), major depressive episodes (Keren et al., Reference Keren, O'Callaghan, Vidal-Ribas, Buzzell, Brotman, Leibenluft and Stringaris2018), or remitted depression (Dichter, Kozink, McClernon, & Smoski, Reference Dichter, Kozink, McClernon and Smoski2012), and may thus represent a common marker for depression-associated reward deficits. However, findings with respect to depression-related alterations during natural reward processing have revealed less consistent results (Oh et al., Reference Oh, Lee, Patriquin, Oldham and Salas2021; Smoski, Rittenberg, & Dichter, Reference Smoski, Rittenberg and Dichter2011). Several electroencephalography (EEG) studies that directly compared processing of monetary and natural rewards in depression suggest not only common but also domain-specific neurofunctional alterations (Ait Oumeziane et al., Reference Ait Oumeziane, Jones and Foti2019; Nelson & Jarcho, Reference Nelson and Jarcho2021). Against this background, we examined if depression is characterized by a dysregulation of the ‘common neural currency’ for rewards by capitalizing on a robust fMRI meta-analytic strategy that allowed us to determine common and separable neurofunctional alterations during natural v. monetary reward processing and during anticipation v. receipt of reward in depression.
We conducted a preregistered coordinate-based meta-analysis according to the latest guidelines (Müller et al., Reference Müller, Cieslik, Laird, Fox, Radua, Mataix-Cols and Eickhoff2018; Page et al., Reference Page, McKenzie, Bossuyt, Boutron, Hoffmann, Mulrow and Moher2021) and included case–control fMRI studies examining the anticipation and receipt of monetary and natural rewards in depression. To facilitate a robust determination, we employed Seed-based d Mapping with Permutation of Subject Images (SDM-PSI), a novel method of performing neuroimaging meta-analyses (Albajes-Eizagirre et al., Reference Albajes-Eizagirre, Solanes, Fullana, Ioannidis, Fusar-Poli, Torrent and Radua2019a). SDM-PSI allows (nearly) unbiased estimation of effect sizes and generation of neurofunctional maps based on both positive and negative differences and hence can produce signed differential effect size maps. In line with the goals of the present study, we employed a three-step approach to meta-analytically determine common and domain-specific reward dysfunctions in depression, including a pooled analysis determining non-specific reward alterations, as well as separate meta-analyses determining common and domain-specific hedonic dysregulations during the receipt of monetary v. natural rewards or the anticipation and receipt of monetary rewards, respectively. We hypothesized domain-general alterations in the ventral striatal reward processing system (reflecting dysregulations in the ‘common neural currency’), but domain-specific alterations in prefrontal systems involved in more complex value and evaluation processes. To further characterize common and separable alterations, we employed a series of connectivity and meta-analytic strategies to distinguish the identified regions on the behavioral, network, and genetic levels.
Methods
Data collection procedures, inclusion criteria, and data extraction
The current pre-registered meta-analysis adhered to the guidelines of conducting coordinate-based neuroimaging meta-analyses (Müller et al., Reference Müller, Cieslik, Laird, Fox, Radua, Mataix-Cols and Eickhoff2018). Procedures, hypotheses, and analyses were pre-registered on the OSF-repository (https://osf.io/ay3r9). A comprehensive literature search was performed based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Page et al., Reference Page, McKenzie, Bossuyt, Boutron, Hoffmann, Mulrow and Moher2021). The search aimed to identify case–control fMRI studies examining reward processing in patients with depression in comparison to healthy controls. For the literature search, biomedical and life science databases including PubMed (https://www.ncbi.nlm.nih.gov/pubmed), Web of Science (https://www.webofscience.com/wos/alldb/basic-search), and PsycInfo (https://www.apa.org/pubs/databases/psycinfo/) were utilized.
Original case–control fMRI studies examining monetary and natural reward processing in depression were extracted and suitable studies from the reference lists of review articles were additionally included. The literature was independently screened based on titles and abstracts by M.C.B and B.K.B. The search terms were: ‘functional magnetic resonance imaging OR fMRI’ AND ‘depression OR major depressive disorder OR unipolar depression OR sub-clinical depression OR at-risk of depression’ in combination with either ‘monetary reward’ OR ‘monetary incentive delay’ OR ‘natural reward’ OR ‘social reward’. Studies written in English, published from January 2001 to December 2021 and reporting whole brain results in standard stereotactic space (Talairach or Montreal Neurological Institute) were included. Only peer-reviewed, original case–control studies comparing patients and healthy controls were included.
Additional exclusion criteria were: (a) studies focusing entirely on participants <18 years and >60 years of age, (b) studies focusing on other mood disorders, including bipolar disorder and postpartum depression, (c) studies reporting region-of-interest (ROI) results only, (d) studies focusing on other reward-related processes such as probabilistic reward learning, prediction error etc., (e) studies reporting findings from an identical dataset as already included studies. The systematic literature review identified 26 suitable neuroimaging studies according to the inclusion criteria, 17 examined monetary reward and 10 examined natural reward (e.g. viewing positive images, positive social feedback, or chocolate) processing in depression.
Aims and coordinate-based meta-analytic implementation
The present case–control neuroimaging meta-analysis aimed to segregate neurofunctional reward alterations in depression, in particular to disentangle general from domain-specific dysregulations in the domains of (a) monetary v. natural reward processing as well as in the domains of (b) anticipation v. receipt of rewards. In an initial step, general reward processing alterations were examined in an overarching meta-analysis that pooled the data from all studies (analysis 1). Next, our primary meta-analysis determined domain-specific alterations in hedonic processing by means of two separate meta-analyses comparing alterations during the receipt of monetary rewards v. natural rewards in depression (analysis 2). Finally, stage-specific alterations with respect to anticipation v. receipt processing of monetary rewards in depression were disentangled by another meta-analysis (analysis 3). However, the number of studies for anticipation of natural rewards was insufficient to explore domain-specific alterations between natural and monetary reward anticipation. All analyses were implemented in Seed-based d Mapping with Permutation of Subject Images (SDM-PSI) version 6.21 (https://www.sdmproject.com/) a novel and highly robust technique for performing neuroimaging meta-analyses (Albajes-Eizagirre et al., Reference Albajes-Eizagirre, Solanes, Fullana, Ioannidis, Fusar-Poli, Torrent and Radua2019a) (online Supplements).
To ensure independence of all studies in our meta-analysis, the coordinates of each study were put in a separate text file according to the guidelines of conducting meta-analysis using SDM-PSI. All analyses were thresholded at p ⩽ 0.0025 uncorrected, k ⩾ 10 voxels. This threshold was in line with the current meta-analysis and was chosen because it balances Type I and Type II errors (Chavanne & Robinson, Reference Chavanne and Robinson2021; Liu et al., Reference Liu, Klugah-Brown, Zhang, Chen, Zhang and Becker2022). Comparative analyses between monetary and natural reward outcomes were performed using Statistical Parametric Mapping (SPM12) software package (https://www.fil.ion.ucl.ac.uk/spm/). The analysis aims to find any common and separate areas of activation between the two identified clusters for natural and monetary reward alterations. We subjected the corresponding two statistical maps of NifTI images (of meta-analytic results of monetary reward outcome and natural reward outcome) to a direct comparison by means of the ImCalc function as implemented in SPM12 (online Supplements).
Characterization of domain-specific hedonic dysregulations on the network, behavioral, genetic and receptor level
Given that our primary meta-analysis revealed distinct neurofunctional alterations during processing of monetary and natural rewards, we further characterized the identified signatures on the functional and neurobiological levels. Network analyses were performed using the Neurosynth database (Yarkoni, Poldrack, Nichols, Van Essen, & Wager, Reference Yarkoni, Poldrack, Nichols, Van Essen and Wager2011) to determine whether the identified regions represent nodes of separable networks. This connectivity analyses were performed using peak coordinates of the caudate (MNI 10/10/4) and putamen (MNI22/8/4). Additional seed-to-whole-brain functional connectivity analyses from an independent dataset were conducted to provide a more fine-grained mapping of the common and separable intrinsic network organization of the identified striatal subregions (Zhao et al., Reference Zhao, Ma, Geng, Zhao, Zhou, Wang and Becker2019). Second, distinct behavioral functions of the regions were identified through meta-analytic topic mapping using peak coordinates of the identified brain regions from the Brain annotation toolbox (BAT) (Liu et al., Reference Liu, Rolls, Liu, Zhang, Yang, Du and Feng2019). Third, to examine separable genetic underpinnings of the identified signatures, we examined genetic expressions of these regions using the Brain annotation toolbox (BAT). This determined which genes had the highest densities and separable expressions in the identified regions (see online Supplements).
Sensitivity, heterogeneity, and publication bias
Inter-study heterogeneity for each cluster was examined by the I2 index which represents the proportion of the total variation caused by study heterogeneity (Higgins & Thompson, Reference Higgins and Thompson2002). Generally, I2 > 50% indicates substantial heterogeneity (Martins et al., Reference Martins, Rademacher, Gabay, Taylor, Richey, Smith and Paloyelis2021). Jackknife sensitivity analysis (repeating the meta-analysis after discarding one study each time) assessing the robustness of the meta-analysis was conducted on Anisotropic Effect Size-Signed Differential Mapping (AES-SDM). Funnel plots (asymmetrical funnel plots indicate that results were driven by few studies or studies with a small sample size, while symmetric plots suggest otherwise) and Egger's tests checked for publication bias (an asymmetric plot and p < 0.05 were recognized as significant).
Exploratory analyses of potential confounders – linear model analysis
A linear model analysis was conducted to determine the potential effects of medication on depression related neurofunctional alterations during monetary and natural reward processing. Studies with medicated and studies with non-medicated individuals were coded as 1 and 0, respectively. Moreover, we repeated the meta-analysis including only data from studies with unmedicated individuals to confirm the stability of the results, i.e., whether the results remain stable without individuals receiving medication. Previous studies have shown that age and gender affect reward-related brain activity (Greimel et al., Reference Greimel, Bakos, Landes, Töllner, Bartling, Kohls and Schulte-Körne2018). Meta-regressions were performed to explore the influence of other potential confounders.
Results
The comprehensive literature search resulted in 26 suitable original fMRI studies on reward processing in depression, i.e., 17 monetary reward studies and 10 natural reward studies (8-social reward and 2-food reward), with n = 1277 participants (n = 633 patients, n = 644 healthy controls). The database included patient data from monetary (n = 391, mean age = 31.47, s.d. = 8.41) and natural reward studies (n = 242, mean age = 35.04, s.d. = 10.26). There were no significant differences in age (p = 0.21, t = 2.07) and gender (p = 0.97, t = 2.10). Healthy controls data from monetary reward studies (n = 393, mean age = 30.95, s.d. = 7.89) and natural reward studies (n = 251, mean age = 31.9, s.d. = 7.98) had no significant differences in age (p = 0.69, t = 2.08) and gender (p = 0.53, t = 2.26). Figure 1 shows the flow diagram of the selection process. Demographic information of the included studies is displayed in Table 1 (online Supplementary Table S1 – characteristics of included studies and online Supplementary Table S2 – PRISMA checklist).
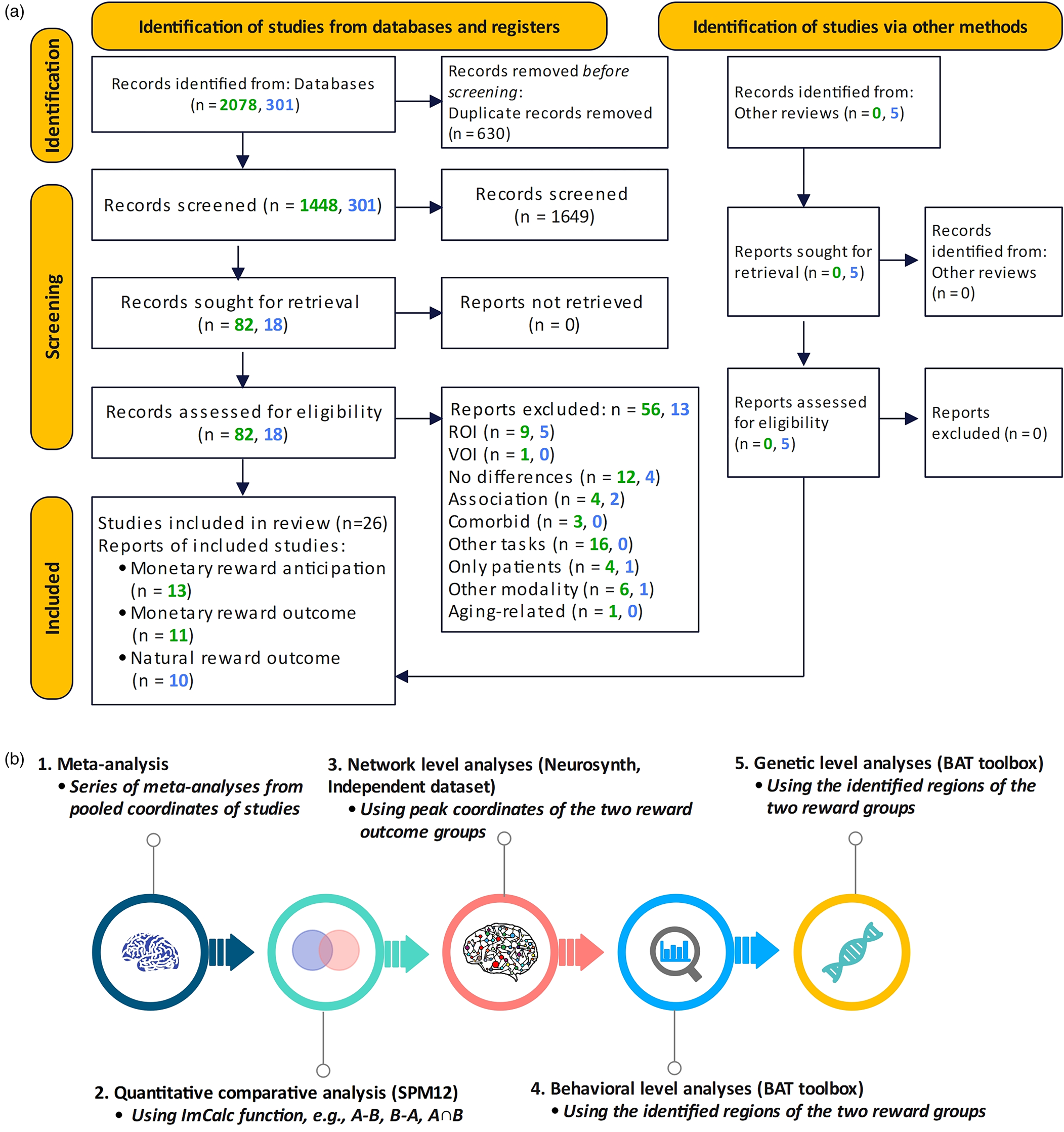
Figure 1. Flow diagram of the systematic literature search and identification of suitable original studies.
Table 1. Demographic information of included studies

NA, not available.
N/F Total number of participants/Total number of female participants, Mean_p mean age of patients, Mean_c mean age of controls, MIDT monetary incentive delay task, s.d. standard deviation, SIDT social incentive delay task.
a The coordinates of this study were merged into one text file when performing the general reward meta-analysis to preserve its independence.
Neurofunctional reward alterations in depression
Analysis 1: General reward processing alterations in depression
Meta-analytic results obtained from the pooled studies onmonetary and natural reward outcome revealed decreased activation in the right striatum, with the cluster encompassing mainly the putamen and extending into the caudate, and the subgenual anterior cingulate in depression (Fig. 2). Between-study heterogeneity and publication bias are displayed in Table 2 (online Supplementary Fig. S1-funnel plots).
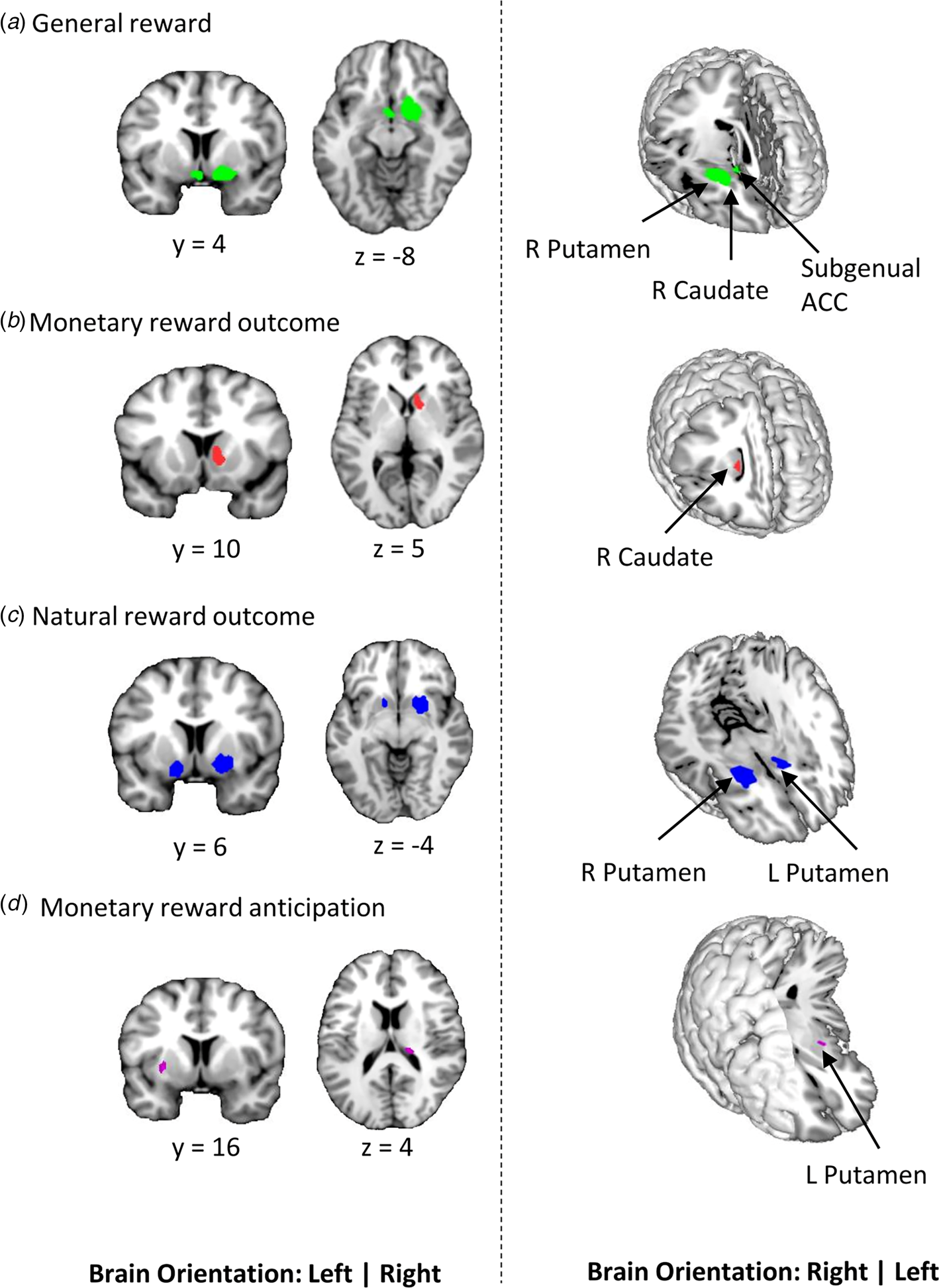
Figure 2. Results of the primary meta-analyses on dysfunctional reward processing in depression. a Display of regions that showed generally reduced reactivity during reward processing in depression as compared to healthy controls. Subsequent meta-analyses revealed regions that are specifically dysfunctional during the hedonic processing of b monetary and c natural rewards. d shows region with altered activation in depression during the anticipation of monetary rewards (p < 0.0025 uncorrected, k ⩾ 10 voxels).
Table 2. Whole-brain meta-analysis results of fMRI studies in unipolar depression at p < 0.0025 uncorrected threshold

ACC, anterior cingulate cortex; fMRI, functional magnetic resonance imaging; HC, healthy controls; MNI, Montreal Neurological Institute; L, left hemisphere; R, right hemisphere; SDM, seed-based d mapping.
Analysis 2: Domain-specific (monetary v. natural) reward processing alterations in depression
During monetary reward outcome, depressed patients exhibited decreased activation in the right caudate compared to healthy controls (Fig. 2b). Depressed patients exhibited decreased activation in more dorsal parts of the striatum with the clusters being specifically located in the bilateral putamen during natural reward outcome (Fig. 2c). For a more fine-grained localization of the monetary v. natural reward processing alterations, additional mapping using the Human Brainnetome Atlas (Fan et al., Reference Fan, Li, Zhuo, Zhang, Wang, Chen and Jiang2016) was employed. Peak coordinates of the monetary outcome alterations (MNI 10/10/4) mapped onto the ventral caudate whereas coordinates for the natural reward alterations (MNI 22/8/-4) mapped onto the ventromedial putamen supporting a ventral to dorsal striatum dissociation of the alterations (Zhao et al., Reference Zhao, Ma, Geng, Zhao, Zhou, Wang and Becker2019; Zhou et al., Reference Zhou, Zimmermann, Xin, Zhao, Derckx, Sassmannshausen and Becker2019).
Analysis 3: Stage-specific (anticipation v. outcome) reward processing alterations in depression
There was decreased activation in the right thalamus and left putamen during reward anticipation (Fig. 2d) in depressive individuals relative to controls. Patients displayed decreased activation in the right caudate during monetary reward outcome. There were no regions of increased activation across all four meta-analyses.
Domain-specific (natural v. monetary) reward dysregulations: regional comparative, network level, and behavioral characterization
According to the aims of the present study, comparative analyses were implemented to further determine common v. separable regions of altered monetary v. natural reward outcome processing in depression. The direct comparison did not reveal an overlap between neural alterations in the two domains. It confirmed that decreased monetary outcome activity was located in the right caudate. In contrast, decreased natural outcome alterations were located in more dorsal regions of the striatum, i.e., the bilateral putamen (Fig. 3a).
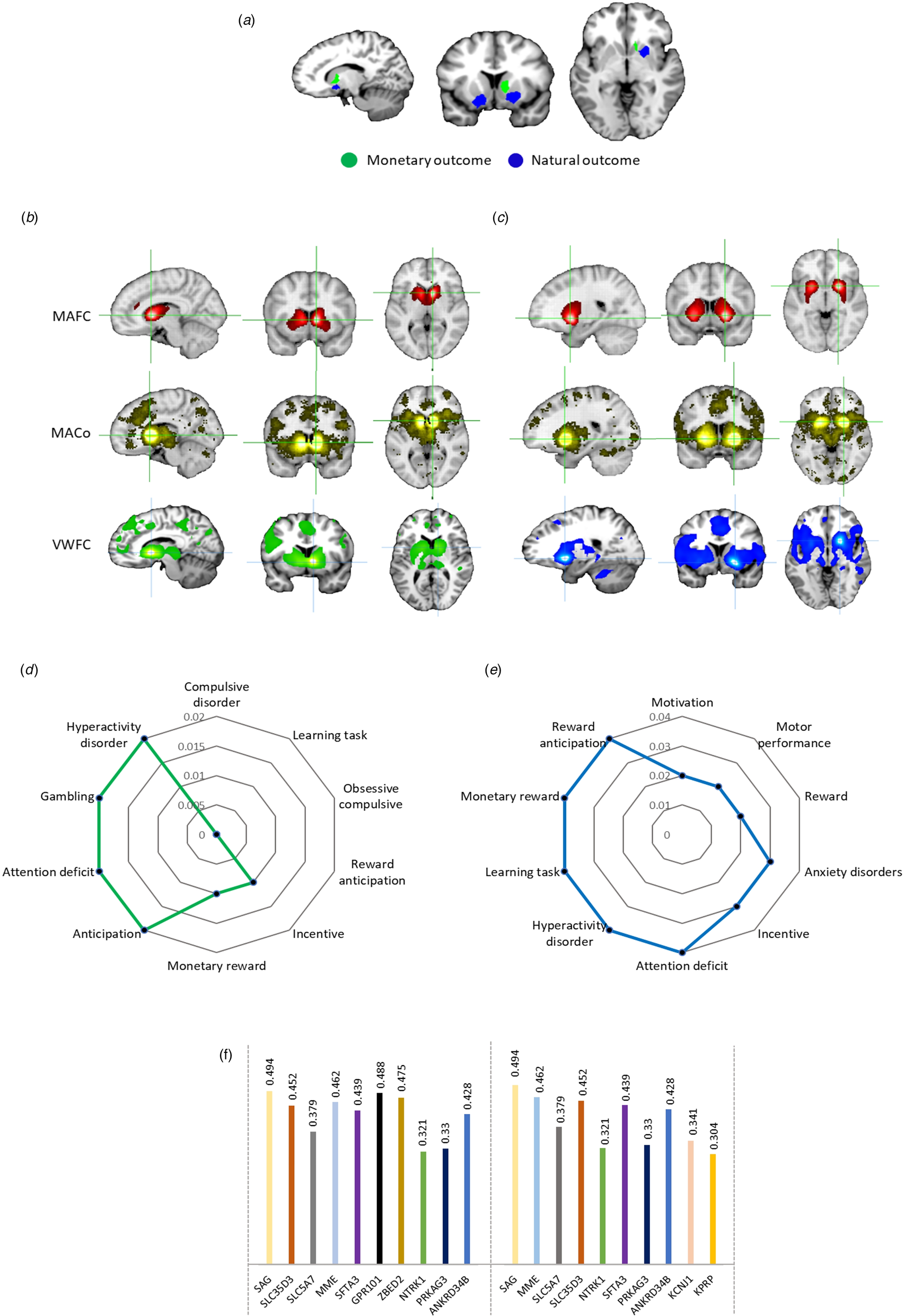
Figure 3. Regional, behavioral, network, and molecular level characterization of the monetary and natural reward outcome alterations in depression. a Comparative analysis of monetary v. natural outcome alterations in depression. Further meta-analytic and voxel-wise characterization of the identified regions using Neurosynth and independent resting state fMRI data. b Brain networks of the identified caudate region of monetary reward outcome alterations. c Brain networks of the identified putamen region of natural reward outcome alterations. MAFC, meta-analytic functional connectivity; MACo, meta-analytic co-activation; VWFC, voxel-wise functional connectivity. d Meta-analytic topic mapping of the right caudate. e Meta-analytic topic mapping of the right putamen. The terms are arranged and read from top in clockwise direction according to p values. f Genetic expression of the caudate (left side) and putamen (right side). The plot shows the genetic symbols according to their respective correlation values.
Network-level and co-activation characterization of the identified striatal regions for monetary (Fig. 3b) and natural reward dysregulations (Fig. 3c) revealed functional connectivity, meta-analytic co-activation, and voxel-wise functional connectivity maps. The caudate region that was identified for monetary reward outcome dysfunctions in depression coupled with core regions of the mesocorticolimbic pathways, including bilateral ventral striatal regions, ventral tegmental area (VTA) and cortical midline structures, while the putamen region that was identified for natural reward outcome dysfunctions in depression coupled stronger with bilateral dorsal striatal regions as well as lateral frontal and insular regions (online Supplementary Fig. S2).
Examination of the top 10 behavioral terms from the behavioral analyses revealed that both regions were characterized by reward, motivation and emotional arousal associated terms, while the caudate region was strongly related to decision making and compulsive behavior and the putamen region was stronger involved in motor and behavioral control (Fig. 3d and Fig. 3e, stronger associations are depicted as smaller p values).
Genetic level characterization
Genetic expression analyses revealed that S-antigen visual arrestin (SAG) was the most expressed gene in both caudate and putamen. The top ten genes were generally highly similar (Fig. 3f).
Heterogeneity, publication bias and sensitivity
The results of tests for heterogeneity (I2), approximate effect sizes (Hedge's g), Egger's probabilities of publication bias as well as jackknife sensitivity results are presented in Table 2. There was low heterogeneity across the main meta-analyses. Publication bias failed to reach statistical significance.
Exploratory analyses
Additional control analyses revealed no consistent or strong evidence for the influence of potential confounders i.e., medication, age, and gender (online Supplements).
Discussion
Depressive mood and anhedonia represent key symptoms of major depression and have been associated with neurobiological dysregulations in reward and motivational processes. Many studies have utilized case–control fMRI designs to map the neurofunctional basis of reward dysregulations in individuals with depression. Several of these studies revealed altered neural activity in the mesocorticolimbic reward circuits in depression. However, it currently remains unclear whether the neurofunctional alterations vary as a function of the reward domain, i.e., natural rewards v. monetary rewards. We hereby conducted a pre-registered neuroimaging meta-analysis to systematically determine common and separable reward dysfunctions in depression. Quantitative analyses of 26 suitable neuroimaging studies focusing on reward-related processes in 633 patients and 644 controls revealed that the right striatum and subgenual ACC exhibit generally reduced reward reactivity in patients with depression, with subsequent comparative analysis demonstrating that more ventral parts of the right striatum (caudate) show reduced reactivity during monetary reward outcome while more dorsal and bilateral parts of the striatum (putamen) show reduced reactivity during the receipt of natural rewards. Additional meta-analytic characterization and an independent fMRI dataset revealed that the two identified regions exhibit common as well as separable characteristics. On the network level, the caudate interacted with ventral striatum, ventral tegmental area (VTA), and cortical midline structures while the putamen exhibited stronger connectivity with more dorsal striatal and lateral fronto-insular regions. On the behavioral and molecular level, both regions were characterized by similar genetics and an involvement in reward-related processes. However, the putamen showed a stronger involvement in cognitive and motor control related processes. Finally, an exploratory meta-analytic examination of stage-specific reward alterations yielded decreased activation in the right thalamus and left putamen during monetary reward anticipation in depression.
Common alterations – general hedonic reward processing alterations in depression
The current meta-analysis revealed that the subgenual ACC and right putamen extending to the caudate, exhibited decreased reactivity in depression during general processing of reward. Our results align with several original studies that have demonstrated altered brain activity in these regions during reward processing in depression. The striatum has been associated with both, monetary and natural reward processing in healthy individuals (Pizzagalli et al., Reference Pizzagalli, Holmes, Dillon, Goetz, Birk, Bogdan and Fava2009) and dysfunctional reward processing in this region has been reported in several mental disorders, including depression, schizophrenia, and addiction (Klugah-Brown et al., Reference Klugah-Brown, Di, Zweerings, Mathiak, Becker and Biswal2020; Pizzagalli et al., Reference Pizzagalli, Holmes, Dillon, Goetz, Birk, Bogdan and Fava2009; Zimmermann et al., Reference Zimmermann, Kendrick, Scheele, Dau, Banger, Maier and Becker2019).
The subgenual ACC has been consistently involved in reward processing (Rogers et al., Reference Rogers, Ramnani, Mackay, Wilson, Jezzard, Carter and Smith2004), as well as regulatory control in cognitive and emotional domains (Critchley, Reference Critchley2005; Drevets, Savitz, & Trimble, Reference Drevets, Savitz and Trimble2008b), and may serve an integrative function to evaluate rewards and guide the most appropriate choices between rewards (Holroyd & Yeung, Reference Holroyd and Yeung2012). Recent neuropathological models suggest a key role of the subgenual ACC in mood disorders including depression (Drevets, Price, & Furey, Reference Drevets, Price and Furey2008a). It is described as an interface system between the cognitive and emotional networks of the brain that conveys emotional information (Greicius et al., Reference Greicius, Flores, Menon, Glover, Solvason, Kenna and Schatzberg2007). A previous meta-analysis has also shown decreased volume of the subgenual ACC in mood disorders (Hajek, Kozeny, Kopecek, Alda, & Höschl, Reference Hajek, Kozeny, Kopecek, Alda and Höschl2008). Reduced activation in this region is distinctively associated with depression as it cuts across behavioral, emotional, and cognitive aspects.
Distinct neurofunctional alterations during hedonic processing of natural and monetary rewards
While our findings on the neurofunctional basis of general reward processing alterations in depression align with previous meta-analyses, the key aim of the present meta-analysis was to further segregate neurobiological dysregulations during hedonic processing of different rewards. We hypothesized a common ventral striatal deficit across natural and monetary rewards in depression, however we observed non-overlapping alterations. The ventral caudal regions displayed reduced reactivity during monetary rewards while more dorsal striatal regions showed decreased reactivity toward natural rewards. Both regions have been involved in reward processing across domains (Arsalidou, Vijayarajah, & Sharaev, Reference Arsalidou, Vijayarajah and Sharaev2020) and general reward processing alterations in depression (Keren et al., Reference Keren, O'Callaghan, Vidal-Ribas, Buzzell, Brotman, Leibenluft and Stringaris2018). However, the striatum is organized along a ventral to dorsal axis with respect to different behavioral domains and network-level interactions. The ventral parts of the caudate have been primarily involved in reward, reinforcement and incentive salience processing while the more dorsal parts of the striatum mediate sensorimotor processes and some executive functions such as inhibitory control (Haber, Reference Haber2016; Robbins, Gillan, Smith, de Wit, & Ersche, Reference Robbins, Gillan, Smith, de Wit and Ersche2012; Zhuang et al., Reference Zhuang, Xu, Zhou, Yao, Zheng, Zhou and Becker2021). In line with this functional specialization of the striatum subregions, the identified caudate region exhibited strong connections with ventral parts of the striatum, the VTA and cortical midline structures strongly involved in reward-related processing while the putamen region coupled with dorsal striatal regions as well as lateral frontal and insular regions involved in interoceptive processes, pain empathy and regulatory control (Craig, Reference Craig2009; Zhou et al., Reference Zhou, Zimmermann, Xin, Scheele, Dau, Banger and Becker2018; Zhuang et al., Reference Zhuang, Xu, Zhou, Yao, Zheng, Zhou and Becker2021). The network level differentiation was additionally mirrored on the behavioral level. Both regions contributed to reward processes, but the more ventral region was also involved in compulsivity, learning, and motivational processes while the dorsal region showed additional involvement in motor and behavioral control processes. Together, the neurofunctional, network, and behavioral characterization may indicate that, (1) different neurobiological mechanisms underlie depression-related reward dysregulations in the hedonic processing of monetary and natural rewards, and (2) a limited generalization and ecological validity of the prevailing reward paradigms incorporating small amounts of monetary incentives to situations in everyday life that engage primarily natural rewards.
On the molecular level, both regions exhibited a similar genetic profile with S-antigen visual arrestin (SAG), SLC35D3, and MME showing high expressions. SAG has been associated with the G-protein-coupled receptor reactivity and thus sensitivity to several neurotransmitters. SLC35D3 gene has been associated with mid-brain and striatal dopamine signaling (Zhang et al., Reference Zhang, Hao, Li, Zang, Zhao, Li and Li2014) while MME drives the inactivation of neuropeptides like oxytocin (Sterchi, Naim, Lentze, Hauri, & Fransen, Reference Sterchi, Naim, Lentze, Hauri and Fransen1988). Dopamine and oxytocin have been associated with network-level modulation of striatal circuits across reward domains (Grimm et al., Reference Grimm, Kopfer, Küpper-Tetzel, Deppert, Kuhn, de Greck and Reif2020; Scheele et al., Reference Scheele, Wille, Kendrick, Stoffel-Wagner, Becker, Güntürkün and Hurlemann2013; Zhao et al., Reference Zhao, Ma, Geng, Zhao, Zhou, Wang and Becker2019) and this may shape context-dependent reward dysregulations in depression.
Stage-specific neurofunctional reward processing alterations in depression – anticipation v. outcome
While the outcome of rewards is commonly considered a hedonic process, anticipation of rewarding outcomes is mostly considered a motivational process. Dysregulated motivational and approach tendencies toward pleasant events have long been associated with depression (Davidson, Reference Davidson1998). However, previous findings in depression have remained largely inconsistent with respect to the anticipation of rewards. For instance, depressed patients have demonstrated elevated activation during monetary reward anticipation in the anterior cingulate (Dichter et al., Reference Dichter, Felder, Petty, Bizzell, Ernst and Smoski2009; Knutson, Bhanji, Cooney, Atlas, & Gotlib, Reference Knutson, Bhanji, Cooney, Atlas and Gotlib2008) while other studies reported a relatively decreased anticipatory signal in striatal regions including the bilateral caudate, bilateral putamen, ventral striatum, and globus pallidus (Keren et al., Reference Keren, O'Callaghan, Vidal-Ribas, Buzzell, Brotman, Leibenluft and Stringaris2018; Stringaris et al., Reference Stringaris, Vidal-Ribas Belil, Artiges, Lemaitre, Gollier-Briant, Wolke and Paillère-Martinot2015; Takamura et al., Reference Takamura, Okamoto, Okada, Toki, Yamamoto, Ichikawa and Yamawaki2017). Partly resembling these previous findings, we observed decreased anticipatory activity during monetary rewards in the thalamus and putamen of depressed patients. The thalamus represents an early detection node for salient stimuli (Cho et al., Reference Cho, Fromm, Guyer, Detloff, Pine, Fudge and Ernst2013). Decreased striatal activity during anticipation of reward has been reported in depression (Pizzagalli et al., Reference Pizzagalli, Holmes, Dillon, Goetz, Birk, Bogdan and Fava2009), plus other mental disorders (Leroy et al., Reference Leroy, Amad, D'Hondt, Pins, Jaafari, Thomas and Jardri2020; Luijten, Schellekens, Kühn, Machielse, & Sescousse, Reference Luijten, Schellekens, Kühn, Machielse and Sescousse2017) and thus may represent a transdiagnostic marker for deficient motivation.
The present findings have some limitations. First, the exploratory linear model analysis revealed some evidence for potential confounding effects of treatment on the identified region during monetary outcome (Delaveau et al., Reference Delaveau, Jabourian, Lemogne, Guionnet, Bergouignan and Fossati2011). Although both reward results remained stable after excluding studies with medication, future studies are required to fully explore effects of treatment on reward dysregulations in depression. Second, some studies mixed unmedicated and medicated patient groups in a single study and this may increase the chance of type II errors (Dichter et al., Reference Dichter, Felder, Petty, Bizzell, Ernst and Smoski2009). Third, the number of studies for natural reward anticipation was insufficient and thus no corresponding meta-analysis was performend. Fourth, the number of studies was moderate, only 10 studies were included in the natural outcome category. Fifth, this meta-analysis focused on comparing monetary and natural rewards unlike previous studies which differentiated primary and secondary rewards (Sescousse et al., Reference Sescousse, Caldú, Segura and Dreher2013), while another study suggested distinguishable electrophysiological neural signatures between monetary, social and food rewards (Banica, Schell, Racine, & Weinberg, Reference Banica, Schell, Racine and Weinberg2022). However, the limited number of case–control fMRI studies on natural reward processing in depression did not allow us to further segregate primary v. secondary or specific secondary reward categories, respectively. It is difficult to determine whether the neurofunctional alterations determined during natural reward processing are held across all types of natural rewards and also which natural reward processing deficits might be specifically relevant to depression. Moreover, monetary and natural rewards may differ in other important aspects relevant for reward processing, including the magnitude of reward. Finally, some included articles did not explicitly present non-significant results and this may affect meta-analytic results (Albajes-Eizagirre, Solanes, & Radua, Reference Albajes-Eizagirre, Solanes and Radua2019b). The present meta-analysis focused on the differential case–control contrasts (reward v. non-reward) provided by the original studies. For specificity of subsequent meta-analysis, it would be helpful if future studies also report results from within-group comparisons.
Summarizing, the present meta-analysis supports the notion of general reward processing deficits in depression. However, the underlying neurofunctional dysregulations vary according to reward type and sub-processes. Previous work has largely focused on monetary rewards to map disrupted reward processing in depression. However, this may have overshadowed deficits in the processing of natural rewards. Natural rewards prevail in everyday life and shape our social interactions and thus dysregulations in this domain may lead to marked impairments in everyday life. The present meta-analysis has revealed distinguishable neurofunctional reward processing deficits in depression, suggesting that natural and monetary reward processing alterations may be rooted in distinct neurobiological dysfunctions.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291723003410
Data availability
Studies included in the meta-analyses have been cited in the manuscript. Coordinates will be made available upon request.
Funding statement
The present study was supported by the National Natural Science Foundation of China (NSFC 82271583; 32250610208) and the China Brain Project (MOST2030, Grant No. 2022ZD0208500).
Competing interests
None.








