Introduction
A number of population-based studies have reported that people with depressive symptoms are at greater risk of mortality (Wulsin et al., Reference Wulsin, Vaillant and Wells1999; Schulz et al., Reference Schulz, Drayer and Rollman2002; Wulsin et al., Reference Wulsin, Evans, Vasan, Murabito, Kelly-Hayes and Benjamin2005; Lasserre et al., Reference Lasserre, Marti-Soler, Strippoli, Vaucher, Glaus, Vandeleur, Castelao, Marques-Vidal, Waeber, Vollenweider and Preisig2016), particularly from cardiovascular disease (CVD) (Laursen et al., Reference Laursen, Munk-Olsen, Nordentoft and Mortensen2007; Wu and Kling, Reference Wu and Kling2016; Correll et al., Reference Correll, Solmi, Veronese, Bortolato, Rosson, Santonastaso, Thapa-Chhetri, Fornaro, Gallicchio, Collantoni, Pigato, Favaro, Monaco, Kohler, Vancampfort, Ward, Gaughran, Carvalho and Stubbs2017). Even patients with sub-clinical levels of depressive symptoms, face increased mortality risk (Russ et al., Reference Russ, Stamatakis, Hamer, Starr, Kivimaki and Batty2012). A robust literature demonstrates that depressive symptoms increase CVD risk in initially healthy people (Cuijpers and Smit, Reference Cuijpers and Smit2002; Rugulies, Reference Rugulies2002; Wulsin and Singal, Reference Wulsin and Singal2003; Nicholson et al., Reference Nicholson, Kuper and Hemingway2006; Van der Kooy et al., Reference Van der Kooy, van Hout, Marwijk, Marten, Stehouwer and Beekman2007; Gan et al., Reference Gan, Gong, Tong, Sun, Cong, Dong, Wang, Xu, Yin, Deng, Li, Cao and Lu2014) and CVD mortality in those with established disease (Barth et al., Reference Barth, Schumacher and Herrmann-Lingen2004; van Melle et al., Reference van Melle, de Jonge, Spijkerman, Tijssen, Ormel, van Veldhuisen, van den Brink and van den Berg2004; Nicholson et al., Reference Nicholson, Kuper and Hemingway2006; Meijer et al., Reference Meijer, Conradi, Bos, Thombs, van Melle and de Jonge2011; Meijer et al., Reference Meijer, Conradi, Bos, Anselmino, Carney, Denollet, Doyle, Freedland, Grace, Hosseini, Lane, Pilote, Parakh, Rafanelli, Sato, Steeds, Welin and de Jonge2013). Many studies have specifically reported an association between depressive symptoms and mortality in older people. Incident (Penninx et al., Reference Penninx, Guralnik, Mendes de Leon, Pahor, Visser, Corti and Wallace1998; Vinkers et al., Reference Vinkers, Stek, Gussekloo, Van Der Mast and Westendorp2004; Teng et al., Reference Teng, Yeh, Lee, Lin and Lai2013), intermittent (Geerlings et al., Reference Geerlings, Beekman, Deeg, Twisk and Van Tilburg2002) and chronic depression (Geerlings et al., Reference Geerlings, Beekman, Deeg, Twisk and Van Tilburg2002; Schoevers et al., Reference Schoevers, Geerlings, Deeg, Holwerda, Jonker and Beekman2009; Teng et al., Reference Teng, Yeh, Lee, Lin and Lai2013) increase all-cause and CVD mortality in this age group. However, these findings are not entirely consistent, with some studies reporting no association (Callahan et al., Reference Callahan, Wolinsky, Stump, Nienaber, Hui and Tierney1998; Cuijpers, Reference Cuijpers2001; Hybels et al., Reference Hybels, Pieper and Blazer2002; McCusker et al., Reference McCusker, Cole, Ciampi, Latimer, Windholz and Belzile2006). Both persistence and severity of depressive symptoms are associated with increased mortality risk in older people (White et al., Reference White, Zaninotto, Walters, Kivimaki, Demakakos, Shankar, Kumari, Gallacher and Batty2015, Reference White, Zaninotto, Walters, Kivimaki, Demakakos, Biddulph, Kumari, De Oliveira, Gallacher and Batty2016).
Extensive evidence also exists for an association between depressive symptoms and inflammation (Raison et al., Reference Raison, Capuron and Miller2006; Irwin and Miller, Reference Irwin and Miller2007; Miller et al., Reference Miller, Maletic and Raison2009; Miller and Raison, Reference Miller and Raison2016). Longitudinal studies have reported an association between depressive symptoms and inflammatory biomarkers in otherwise healthy people (Gimeno et al., Reference Gimeno, Kivimaki, Brunner, Elovainio, De Vogli, Steptoe, Kumari, Lowe, Rumley, Marmot and Ferrie2009; Hamer et al., Reference Hamer, Molloy, de Oliveira and Demakakos2009; Copeland et al., Reference Copeland, Shanahan, Worthman, Angold and Costello2012; Au et al., Reference Au, Smith, Gariepy and Schmitz2015; Zalli et al., Reference Zalli, Jovanova, Hoogendijk, Tiemeier and Carvalho2015; Bell et al., Reference Bell, Kivimaki, Bullmore, Steptoe and Carvalho2017). In addition, several meta-analyses show higher levels of inflammation in depressed patients compared with healthy controls (Howren et al., Reference Howren, Lamkin and Suls2009; Dowlati et al., Reference Dowlati, Herrmann, Swardfager, Liu, Sham, Reim and Lanctot2010; Liu et al., Reference Liu, Ho and Mak2012; Valkanova et al., Reference Valkanova, Ebmeier and Allan2013; Eyre et al., Reference Eyre, Air, Pradhan, Johnston, Lavretsky, Stuart and Baune2016). A recent cumulative meta-analysis confirmed the robust nature of the depression–inflammation relationship, showing evidence of increased circulating interleukin-6 and C-reactive protein (CRP) in depressed compared with non-depressed individuals (Haapakoski et al., Reference Haapakoski, Mathieu, Ebmeier, Alenius and Kivimaki2015). In patients with CVD, the prevalence of depression is four times higher than in people from the general population (Thombs et al., Reference Thombs, Bass, Ford, Stewart, Tsilidis, Patel, Fauerbach, Bush and Ziegelstein2006). Depression also shows a high co-morbidity with a number of major chronic inflammatory or autoimmune disorders, such as rheumatoid arthritis, multiple sclerosis and diabetes type 1 and 2 (Maes et al., Reference Maes, Berk, Goehler, Song, Anderson, Gałecki and Leonard2012). However, it is unclear whether the increased mortality risk in depressed people is due to inflammation.
In light of the well-established role of inflammatory processes in both the pathogenesis of atherosclerosis and the prediction of cardiac events (Shimbo et al., Reference Shimbo, Chaplin, Crossman, Haas and Davidson2005; Golia et al., Reference Golia, Limongelli, Natale, Fimiani, Maddaloni, Pariggiano, Bianchi, Crisci, D'Acierno, Giordano, Di Palma, Conte, Golino, Russo, Calabro and Calabro2014; Biasucci et al., Reference Biasucci, La Rosa, Pedicino, D'Aiello, Galli and Liuzzo2017), it has been proposed that inflammation may be mediating the association between depression and mortality, particularly in relation to CVD. However, evidence linking inflammation and depressive symptoms with the development of CVD is inconsistent. A population-based study reported that depressive symptoms and CRP interact in the prediction of coronary heart disease (CHD) in men (Ladwig et al., Reference Ladwig, Marten-Mittag, Lowel, Doring and Koenig2005). However, other studies have demonstrated that depressive symptoms increase CVD risk independently of inflammatory biomarkers (Empana et al., Reference Empana, Sykes, Luc, Juhan-Vague, Arveiler, Ferrieres, Amouyel, Bingham, Montaye, Ruidavets, Haas, Evans, Jouven and Ducimetiere2005; Nabi et al., Reference Nabi, Singh-Manoux, Shipley, Gimeno, Marmot and Kivimaki2008; Surtees et al., Reference Surtees, Wainwright, Boekholdt, Luben, Wareham and Khaw2008; Davidson et al., Reference Davidson, Schwartz, Kirkland, Mostofsky, Fink, Guernsey and Shimbo2009; Hamer et al., Reference Hamer, Bates and Mishra2011), whilst several studies have reported minimal effects of mediation (Vaccarino et al., Reference Vaccarino, Johnson, Sheps, Reis, Kelsey, Bittner, Rutledge, Shaw, Sopko and Bairey Merz2007; Kop et al., Reference Kop, Stein, Tracy, Barzilay, Schulz and Gottdiener2010; Hiles et al., Reference Hiles, Baker, de Malmanche, McEvoy, Boyle and Attia2015; Hughes et al., Reference Hughes, Patterson, Appleton, Blankenberg, Woodside, Donnelly, Linden, Zeller, Esquirol and Kee2016). To our knowledge, only one study has investigated the combined effect of depressive symptoms and elevated inflammation on CVD development. Ladwig et al. (Reference Ladwig, Marten-Mittag, Lowel, Doring and Koenig2005) showed that combined depressed mood and high CRP (>3 mg/L) conferred a significantly greater risk of future cardiac events than either depressed mood or high CRP alone in older men.
Gender differences have also been observed in the association between late-life depression and mortality. Several studies report a greater mortality risk in men (Anstey and Luszcz, Reference Anstey and Luszcz2002; Ryan et al., Reference Ryan, Carriere, Ritchie, Stewart, Toulemonde, Dartigues, Tzourio and Ancelin2008; Jeong et al., Reference Jeong, Lee, Lee, Park, Huh, Han, Kim, Chin and Kim2013; Diniz et al., Reference Diniz, Reynolds, Butters, Dew, Firmo, Lima-Costa and Castro-Costa2014), particularly for incident depression (Teng et al., Reference Teng, Yeh, Lee, Lin and Lai2013) and CVD (Penninx et al., Reference Penninx, Guralnik, Mendes de Leon, Pahor, Visser, Corti and Wallace1998; Wulsin et al., Reference Wulsin, Vaillant and Wells1999); whilst women appear to have increased mortality risk in the presence of chronic or severe depressive symptoms (Ryan et al., Reference Ryan, Carriere, Ritchie, Stewart, Toulemonde, Dartigues, Tzourio and Ancelin2008; Teng et al., Reference Teng, Yeh, Lee, Lin and Lai2013). An emerging literature also suggests women are more likely to present with inflammation when depressed (Bell et al., Reference Bell, Kivimaki, Bullmore, Steptoe and Carvalho2017), potentially due to higher reactivity to stressful stimuli (Piccinelli and Wilkinson, Reference Piccinelli and Wilkinson2000). Whether the inflammation and depression link is particularly important for women is still unknown.
In this study, we used data from the English Longitudinal Study of Ageing (ELSA) to investigate the combined effects of depressive symptoms and inflammation on CVD and all-cause mortality risk in older men and women. In particular, we sought to investigate whether depressive symptoms moderate the mortality risk associated with increased inflammation or whether inflammation could be mediating the association between depressive symptoms and mortality. In light of previous findings, we hypothesise that people with both depressive symptoms and inflammation will have significantly greater mortality risk than people with depressive symptoms or inflammation alone.
Materials and methods
Study population
The ELSA is a prospective study of a representative sample of community-dwelling people aged 50 and over living in England. It collects health, social and economic data. The study commenced in 2002, and the sample has been followed up every 2 years. Data are collected using computer-assisted personal interviews and self-completion questionnaires, with additional nurse visits for the assessment of biomarkers every 4 years. Wave 1 included a baseline interview and took place in 2002–2003. Wave 2 took place in 2004–2005 and consisted of an interview and a health examination that included a collection of blood samples. At wave 1, the sample consisted of 11 391 study members and was deemed to be nationally representative. For more information on ELSA see http://www.elsa-project.ac.uk/.
Our study included 5328 people, aged 52–89 years, from an initial cohort of 8670 who participated in the interview at wave 2. We excluded 1084 individuals who did not participate in the health examination survey, 134 who did not consent for their vital status data to be included, 1411 who were unable to provide a blood sample and 330 individuals whose CRP values were unavailable or not reliable. The latter included samples which were lost in the post, received later than 5 days after collection, considered unusable by the laboratory or of insufficient amount to be analysed. Detailed information about how the ELSA data were collected and processed into their current format and about how each variable was coded is available at https://discover.ukdataservice.ac.uk/series/?sn=200011. We also excluded 155 participants from the analysis because their CRP levels were ⩾20 ml/L, allowing for the elimination of individuals with acute inflammation, while another 229 participants were excluded because of missing values in the covariates.
Participants included and excluded from the analysis did not differ significantly in terms of sex. The group included in the analysis was younger, less likely to be depressed, more likely to be married, more educated and wealthier than the group who was excluded.
Assessment of inflammation
Blood samples were taken by the study nurse at wave 2 and serum CRP was analysed by Royal Victoria Infirmary, Newcastle. High sensitivity plasma CRP level was dichotomised into two categories: <3 mg/L was defined as normal and 3–20 mg/L was defined as high. This cut off point is based on guidelines from the Centre for Disease Control and Prevention and the American Heart Association, suggesting that plasma CRP values of >3.0 mg/L might be predictive of CVD (Pearson et al., Reference Pearson, Mensah, Alexander, Anderson, Cannon, Criqui, Fadl, Fortmann, Hong and Myers2003).
Assessment of depressive symptoms
Depressive symptoms were measured at wave 2 (2004–2005) using the eight-item Centre for Epidemiological Studies Depression Scale 8 (CES-D8) which is a self-report questionnaire designed to measure depressive symptomatology in the general population (Radloff, Reference Radloff1977). Respondents were asked how often they felt depressed, felt that everything was an effort, slept restlessly, were happy, felt lonely, enjoyed life, felt sad, and could not get going. The two positive items (‘was happy’ and ‘enjoyed life’) are reverse coded, so a higher score here also indicates a more depressed mood. We subsequently derived a summary CES-D score by adding responses to all eight dichotomous questions (possible range: 0–8). The exact wording of the different items can be found in appendix 1. The eight-item abbreviated version of the CES-D has been widely used, is internally consistent, has been validated in both the general population (Van de Velde et al., Reference Van de Velde, Levecque and Bracke2009) and older adults, and shows a comparable construct of depression across 11 different countries (Missinne et al., Reference Missinne, Vandeviver, Van de Velde and Bracke2014). The presence of depressive symptoms was defined as CES-D ⩾4 as per previous publications (Steffick, Reference Steffick2000; Hamer et al., Reference Hamer, Batty and Kivimaki2012; Mhaoláin et al., Reference Mhaoláin, Fan, Romero-Ortuno, Cogan, Cunningham, Kenny and Lawlor2012; Demakakos et al., Reference Demakakos, Cooper, Hamer, de Oliveira, Hardy and Breeze2013; Malgaroli et al., Reference Malgaroli, Galatzer-Levy and Bonanno2017). This conservative threshold has been found to produce comparable results to the >16 cut off on the well-validated 20-item CES-D scale (Radloff, Reference Radloff1977; Steffick, Reference Steffick2000).
Depressive symptoms and inflammation as a combined variable
In order to ascertain whether the combination of depressive symptoms and inflammation predicts mortality, the variables based on wave 2 assessments were combined and four new variables were computed: ‘no depressive symptoms/low inflammation’, ‘no depressive symptoms/high inflammation’, ‘depressive symptoms/low inflammation’ and ‘depressive symptoms/high inflammation’.
Mortality
Mortality was ascertained for a mean 7.7 year period for consenting study members (5328) by linking to the UK National Health Service mortality register up until 12 November 2015. In England, all deaths need to be registered within 5 days, therefore participants not registered as dead were assumed to be alive. Deaths were classified according to International Classification of Diseases (ICD) 10th Edition. Deaths with ICD10 codes I00 to I99 were classified as cardiovascular deaths.
Covariates
All covariates were collected at wave 2 (2004–2005) with the exception of education and sex which were collected at wave 1. All covariates where determined by self-report, with the exception of body mass index (BMI). Age was treated as a continuous variable. Socioeconomic status (SES) was operationalised by using marital status (married/cohabiting v. not married/single/divorced), level of education (degree/higher/A-level, GCSE/O-level/other, no qualifications) and total wealth in tertiles. Total wealth was defined from the sum of financial, physical (e.g. businesses, land) and housing wealth, minus debts and pension payments. Health behaviours included: smoking (never smoked, ex-smoker, current smoker) and BMI (<25 kg/m2, 25–29.99 kg/m2, >30 + kg/m2) (Banks et al., Reference Banks, Breeze, Lessof and Nazroo2006). The presence of chronic diseases were added as separate variables and defined as yes/no. They were calculated as lifetime self-reported physician diagnoses of chronic conditions (i.e. CVD (myocardial infarction and stroke), chronic lung disease, cancers (of any site) and emotional, nervous and psychiatric problems).
The covariates have been included because they have all been shown to be associated with mortality and are therefore potential confounders (Marmot et al., Reference Marmot, Allen, Bell, Bloomer and Goldblatt2012; Wong, Reference Wong2014; Pletcher and Moran, Reference Pletcher and Moran2017). Furthermore, health behaviours such as smoking and BMI were included as they have been shown to mediate the relationship between depression and mortality (Joynt et al., Reference Joynt, Whellan and O'Connor2003).
Statistical analysis
Differences between the characteristics of the participants included and excluded from the analysis were analysed. Baseline characteristics were analysed by depressive symptoms and inflammation levels. The association of depressive symptoms and inflammation levels (separately and in combination) with CVD and all-cause mortality were assessed by Cox proportional hazards regression models. The proportionality assumption was tested using Nelson–Aalen cumulative hazard curves and Schoenfeld residuals. We inspected the plots and the global test (p = 0.8052) confirming that we do not have a violation of the proportional assumption. Survival time was measured in months, from the date of interview in wave 2 (2004–2005) to the date of death or 12 November 2015, whichever was first. Kaplan Meier survival curves are available online (online Supplementary Fig. S1).
We investigated whether there were significant interactions between age or sex and depression/inflammation categories in order to determine whether the association between depression/inflammation levels and mortality varied according to age or between men and women. We used the likelihood ratio test to compare the goodness of fit of a stratified model. We found no interaction between sex and depressive symptoms, however, we did find that the association between inflammation and mortality varied significantly by sex (p value = 0.013). There was also an age interaction between inflammation and mortality, however, this disappeared once we stratified our analysis by sex, therefore, we only present the sex-stratified analyses. We first fitted a basic unadjusted model, which was followed by an age-adjusted model. We then additionally adjusted for socioeconomic variables, health behaviours and chronic diseases.
To investigate moderation we tested whether a multiplicative interaction between depressive symptoms and inflammation was significantly associated with mortality. To investigate mediation, we first examined the association between depressive symptoms and mortality and then added inflammation to see how much of the association was explained. All analyses were performed using STATA 13.0 (StataCorp LP, College Station Texas).
Results
There were 420 all-cause male deaths (including 112 CVD related) over mean of 7.6 years follow-up and 334 all-cause female deaths (including 109 CVD related) over a mean of 7.8 years follow-up. Out of a total of 5328 people, we identified 420 all-cause deaths in men and 334 in women during 18 594 and 22 519 person-years, respectively.
Table 1 represents social-demographic characteristics in depression/inflammation categories in men and women separately. Men and women with concurrent depressive symptoms and high inflammation were more likely to be poorer, less educated, more likely to smoke, have a higher BMI and were more likely to have chronic lung disease and emotional, nervous and psychiatric problems. There were no significant overall differences in the frequency of CVD or cancer between depression/inflammation levels in either men or women. (Table 1).
Table 1. Baseline characteristics of men and women aged 52–89 years by depressive symptoms and inflammation level
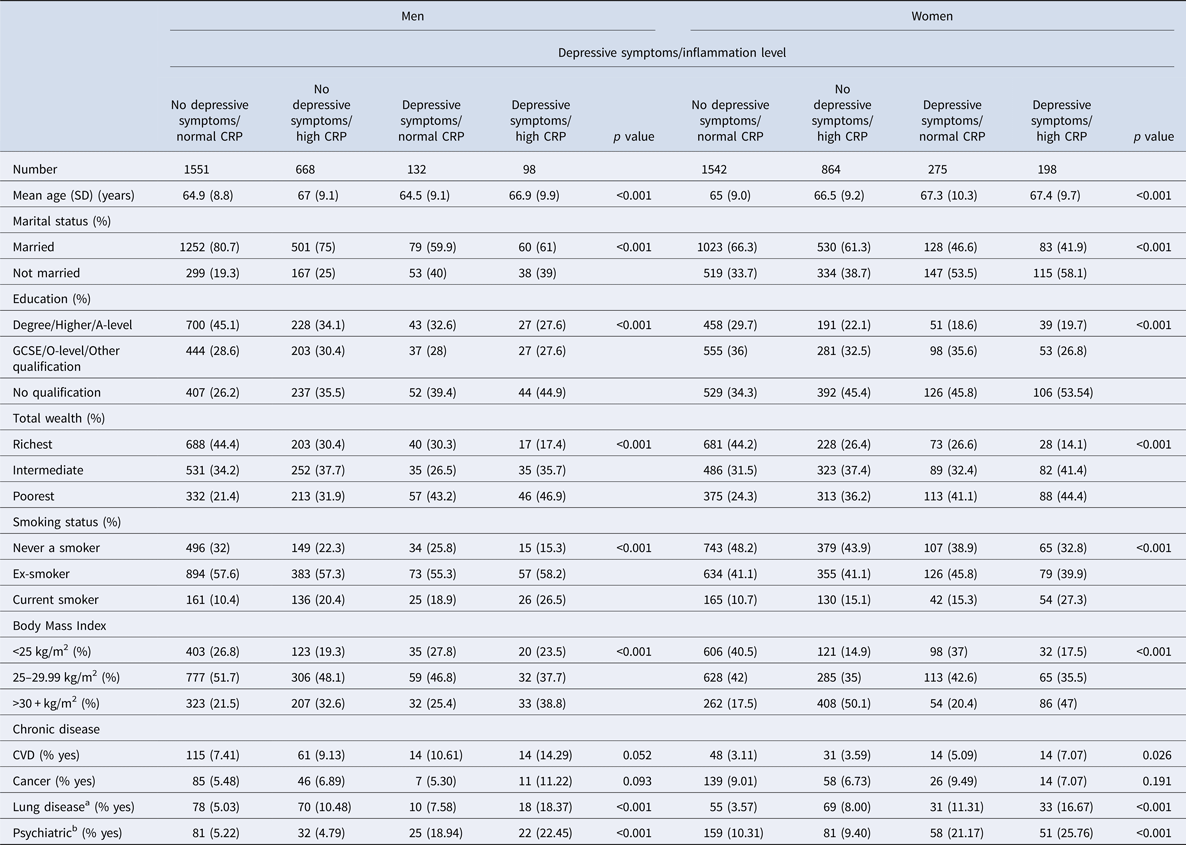
SD = standard deviation. Statistical tests examined the associations of demographic variables with depressive symptoms/inflammation levels. ANOVA tests were used for continuous variables and Chi-square tests were used for categorical variables.
a Chronic lung disease.
b Emotional, nervous or psychiatric diseases.
Depressive symptoms and inflammation as a combined predictor of CVD mortality
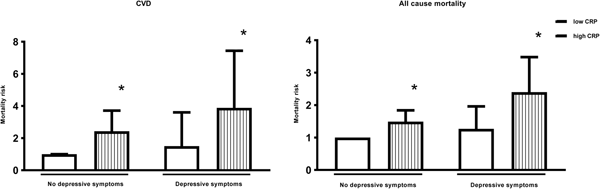
In men, depressive symptoms alone were not associated with any significant increase in the risk of death, whilst high inflammation was associated with a 238% (HR: 3.38; 95% CI 2.23–5.10) increased risk. Men with both depressive symptoms and high inflammation had a 584% (HR: 6.84; 95% CI 3.71–12.6) increased CVD mortality risk. This association remained significant after adjustment for age, SES and health behaviours, with men who had both depressive symptoms and high inflammation demonstrating a 289% (HR: 3.89; 95% CI 2.04–7.44) increased risk of death (p value <0.001) (Table 2 and Fig. 1). In women, the associations were more modest and failed to reach significance (Table 2).

Fig. 1. Adjusted hazard ratios for CVD and all-cause mortality according to levels of depressive symptoms and inflammation in men. * Statistically significant at P<0.001.CVD = Cardiovascular disease; CRP = C-reactive protein. Hazard ratios are adjusted for age, socioeconomic variables (marital status, level of education, total wealth), health behaviours (smoking, body mass index) and chronic diseases (cardiovascular disease, cancers, chronic lung disease and emotional, nervous and psychiatric problems). n = 5,328.
Table 2. Association between depressive symptoms/inflammation levels and all-cause and cardiovascular mortality by sex+
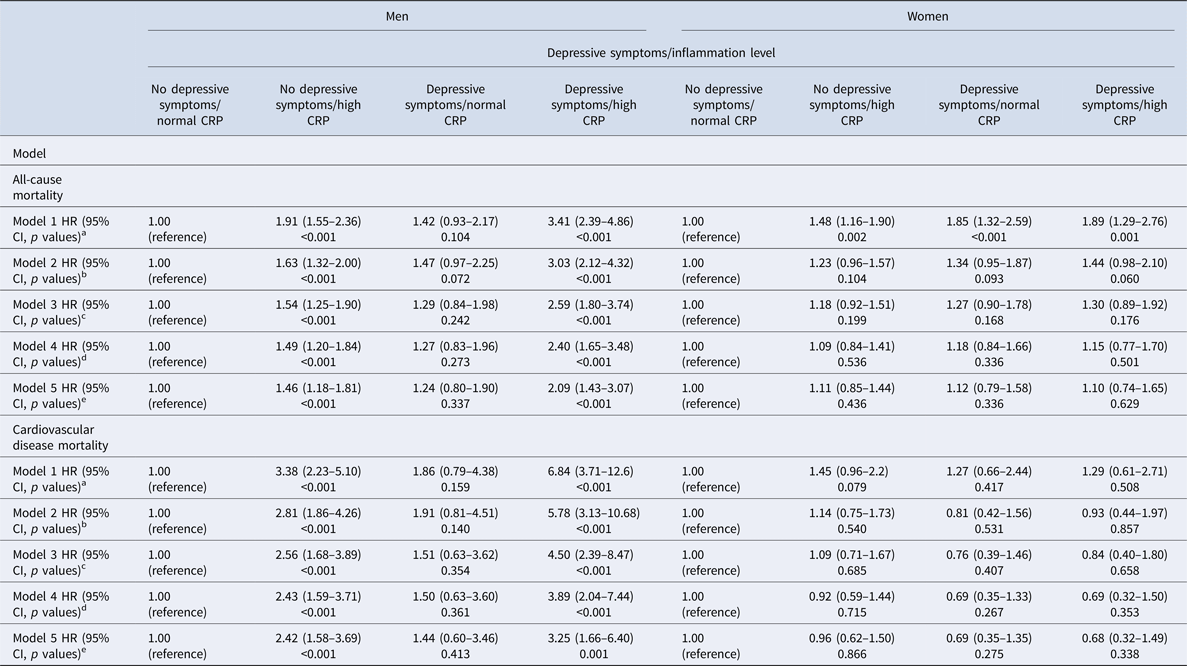
HR = hazard ratio; CI = confidence interval; CRP = C-reactive protein. Cox regression survival analysis models, stratified by sex, are adjusted as follows:
a Inflammation and depressive symptoms as main effects.
b As model 1, plus adjustment for age.
c As model 2, plus adjustment for socioeconomic variables (marital status, level of education, total wealth).
d As model 3, plus adjustment for health behaviours (smoking, body mass index).
e A model 4, plus individually adjustment for chronic diseases (cardiovascular disease, cancers, chronic lung disease and emotional, nervous and psychiatric problems).
Depressive symptoms and inflammation as a combined predictor of all-cause mortality
In men, the direction of results was similar for all-cause mortality. Depressive symptoms alone were not associated with a significant increase in the risk of death, whilst high inflammation was associated with a 91% (HR: 1.91; 95% CI 1.55–2.36) increased risk, compared with men with neither. Men with both depressive symptoms and high inflammation had a 241% (HR: 3.41; 95% CI 2.39–4.86) increased mortality risk. This association was attenuated after adjustment for age, SES and smoking. However, the association remained significant, with men who had both depressive symptoms and high inflammation demonstrating a 140% (HR: 2.40; 95% CI 1.65–3.48) increased risk of death (Fig. 1).
The results for women were different. In the unadjusted model, both depressive symptoms and high inflammation separately increased the risk of death by 85% (HR: 1.85; 95% CI 1.32–2.59) and 48% (HR: 1.48; 95% CI 1.16–1.90), respectively. The combination of both depressive symptoms and high inflammation increased the risk to 89% (HR: 1.89; 95% CI 1.29–2.76), only marginally more than depressive symptoms alone. The increased risk of depressive symptoms and high inflammation to all-cause mortality in women was explained by age as after adjustments the risk was no longer significant (Table 2).
Effects of moderation and mediation
Moderation analysis showed that an interaction term of depressive symptoms by inflammation was not significantly associated with mortality in all categories: all-cause mortality in men (p = 0.426); all-cause mortality in women (p = 0.155); CVD mortality in men (p = 0.868) and CVD mortality in women (p = 0.481). This suggests that the mortality risk conferred by increased levels of inflammation is not further augmented by depressive symptoms.
Mediation analysis showed that the strength of the association between depressive symptoms and mortality was not reduced by including inflammation, suggesting a direct effect of depressive symptoms on mortality risk (online Supplementary Table S1).
Discussion
In this study, we examined the combined effect of depressive symptoms and inflammation on CVD and all-cause mortality in a large cohort of older adults. Our findings suggest that older men, with both depressive symptoms and high levels of inflammation, have an increased risk of CVD and all-cause mortality compared with men with depressive symptoms or inflammation alone. In addition, our study demonstrates the independent effects of depressive symptoms and inflammation on mortality, finding no evidence of either moderation or mediation. To our knowledge, our study is the first to investigate the combined effect of depressive symptoms and inflammation of mortality in both men and women.
Depression, inflammation and mortality
We demonstrated an increased risk of all-cause mortality in the comparison between men with high and low baseline levels of inflammation. The addition of depression to the model increased the risk substantially suggestive of a particularly high-risk phenotype in men. This supports findings in healthy, older men showing that high inflammation predicted cardiovascular events only in people with depressed mood (Ladwig et al., Reference Ladwig, Marten-Mittag, Lowel, Doring and Koenig2005). These findings suggest that depression and inflammation might cause CVD through separate physiological pathways, such as elevated interleukin-6 upstream of CRP (Ridker, Reference Ridker2016), triglycerides (Parekh et al., Reference Parekh, Smeeth, Milner and Thure2017), cortisol as a result of stress-induced hyperactivity of the hypothalamic-pituitary-adrenal axis (Jokinen and Nordstrom, Reference Jokinen and Nordstrom2009), endothelial dysfunction (Chen et al., Reference Chen, Yiu and Tse2011) and platelet activation (Williams et al., Reference Williams, Rogers, Wang and Ziegelstein2014).
Our study found no significant interaction between depressive symptoms and inflammation on mortality and no effect of mediation, a finding which is supported by other studies but not by all. To our knowledge, there is only one other study which looked at the potential synergistic effect of depressive symptoms and inflammation in the prediction of cardiovascular events. Ladwig showed a significant interaction between depressive symptoms and inflammation in the prediction of cardiovascular events, suggesting a shared underlying mechanism (Ladwig et al., Reference Ladwig, Marten-Mittag, Lowel, Doring and Koenig2005). These authors, however, have only investigated men.
Most previous studies investigating the effects of inflammation mediating the association between depression and all-cause or CVD mortality showed either no or small effects. Similar to our study, Empana et al., (Reference Empana, Sykes, Luc, Juhan-Vague, Arveiler, Ferrieres, Amouyel, Bingham, Montaye, Ruidavets, Haas, Evans, Jouven and Ducimetiere2005) showed that men with depressive symptoms had a 53% increase in the odds of CHD and the association remained unchanged when inflammatory markers were added to the model. Davidson et al., Reference Davidson, Schwartz, Kirkland, Mostofsky, Fink, Guernsey and Shimbo2009 found that depressive symptoms increased the risk of incident CHD; this risk was not explained by increased inflammation in either men or women. Nabi et al., Reference Nabi, Singh-Manoux, Shipley, Gimeno, Marmot and Kivimaki2008 also found an association between psychological distress and incident CHD which remained after adjustment for inflammatory markers. Contrary to our study and the studies cited above, there has also been reports of mediation. Hughes et al., (Reference Hughes, Patterson, Appleton, Blankenberg, Woodside, Donnelly, Linden, Zeller, Esquirol and Kee2016) reported an association between depressive symptoms and all-cause mortality which was partly explained by inflammation (CRP 7.3%). Inflammation partly mediated the predictive value of depressive symptoms by 6.5% in cardiovascular mortality risk (Kop et al., Reference Kop, Stein, Tracy, Barzilay, Schulz and Gottdiener2010), and by 8.1% in cardiovascular hospitalization (Hiles et al., Reference Hiles, Baker, de Malmanche, McEvoy, Boyle and Attia2015). This supports the knowledge that other pathways are also involved in the depression and mortality link.
Effect of gender on the link between depressive symptoms, inflammation and mortality
Clear sex-specific differences were observed in the inter-relationships of depressive symptoms, inflammation and mortality in this study. The combination of depressive symptoms and inflammation only conferred an increased mortality risk in men. A previous population cohort study investigating inflammation and mortality also showed sex differences. Ahmadi-Abhari et al., reported that high levels of CRP increased mortality risk in men at lower clinical threshold categories than in women, most notably for CVD mortality (2013). A similar trend has also been observed in the development of CHD. In a study of older men and women, Reference Cushman, Arnold, Psaty, Manolio, Kuller, Burke, Polak and TracyCushman et al. showed that the presence of high inflammation was predictive of CHD in men with intermediate Framingham Risk Scores, whereas it only became predictive of CHD in women with a high Framingham risk (2005). Furthermore, a large meta-analysis showed that inflammation only discriminated 10-year risk of cardiovascular events in men, but not in women (Kaptoge et al., Reference Kaptoge, Di Angelantonio, Pennells, Wood, White, Gao, Walker, Thompson, Sarwar, Caslake, Butterworth, Amouyel, Assmann, Bakker, Barr, Barrett-Connor, Benjamin, Bjorkelund, Brenner, Brunner, Clarke, Cooper, Cremer, Cushman, Dagenais, D'Agostino, Dankner, Davey-Smith, Deeg, Dekker, Engstrom, Folsom, Fowkes, Gallacher, Gaziano, Giampaoli, Gillum, Hofman, Howard, Ingelsson, Iso, Jorgensen, Kiechl, Kitamura, Kiyohara, Koenig, Kromhout, Kuller, Lawlor, Meade, Nissinen, Nordestgaard, Onat, Panagiotakos, Psaty, Rodriguez, Rosengren, Salomaa, Kauhanen, Salonen, Shaffer, Shea, Ford, Stehouwer, Strandberg, Tipping, Tosetto, Wassertheil-Smoller, Wennberg, Westendorp, Whincup, Wilhelmsen, Woodward, Lowe, Wareham, Khaw, Sattar, Packard, Gudnason, Ridker, Pepys, Thompson and Danesh2012). Some reports on the association between depression and inflammation also support our findings, although not all. Two large population cohort studies have shown that major depression/depressive symptoms and inflammation are more strongly associated in men than in women (Ford and Erlinger, Reference Ford and Erlinger2004; Elovainio et al., Reference Elovainio, Aalto, Kivimaki, Pirkola, Sundvall, Lonnqvist and Reunanen2009). However in contrast, in a study of 508 healthy adults, depressive symptoms were only associated with inflammation in women, not in men (Ma et al., Reference Ma, Chiriboga, Pagoto, Rosal, Li, Merriam, Hebert, Whited and Ockene2010). Furthermore, a study of women with suspected coronary ischemia demonstrated a robust association between depressive symptoms and inflammation which was not explained by CVD risk factors (Vaccarino et al., Reference Vaccarino, Johnson, Sheps, Reis, Kelsey, Bittner, Rutledge, Shaw, Sopko and Bairey Merz2007).
Further speculation on gender differences is inspired by a recent review by Raison and Miller (Reference Raison and Miller2017). The authors propose that in evolutionary terms, depression may have provided an adaptive advantage to women. Inflammation was detrimental to fertility in ancestral environments (Van Bodegom et al., Reference Van Bodegom, May, Meij and Westendorp2007; Schaller and Park, Reference Schaller and Park2011; Kobayashi et al., Reference Kobayashi, Uejyo, Oyama, Rahman and Kumura2013). Depressive symptoms promoted sickness behaviours (e.g. lethargy, psychomotor slowing and social withdrawal) which provided increased protection against pathogens (e.g. by conserving energy for an immune response), thereby reducing the need for a high inflammatory response. This is supported by findings which show that women demonstrate increased levels of depression in response to inflammatory challenge compared with men (Moieni et al., Reference Moieni, Irwin, Jevtic, Olmstead, Breen and Eisenberger2015; Udina et al., Reference Udina, Castellví, Moreno-España, Navinés, Valdés, Forns, Langohr, Sola, Vieta and Martín-Santos2012). If women are more likely to develop depression in response to immune activation then it is possible that the presence of depressive symptoms is likely to reflect less severe underlying biological pathology compared with men and consequently lower mortality risk. Whilst this explanation is intuitively appealing, further research is required to confirm gender specific immune mechanisms in depression.
Another possible explanation for our observed sex differences is the influence of sex hormones, particularly the protective effect of oestrogen on the heart. In people under 75, the incident of cardiovascular-related death is lower in women than in men (British Heart Foundation, 2014), with the development of atherosclerosis occurring post menopause in 95% of women (Fairweather, Reference Fairweather2014). Even at 65–74 years of age, almost two decades after the average occurrence of menopause, women have a substantially lower incidence of CVD compared with men. This suggests that former exposure to endogenous estrogen may be atheroprotective long after menopause. Women have also been shown to have longer telomeres than men (Gardner et al., Reference Gardner, Bann, Wiley, Cooper, Hardy, Nitsch, Martin-Ruiz, Shiels, Sayer, Barbieri, Bekaert, Bischoff, Brooks-Wilson, Chen, Cooper, Christensen, De Meyer, Deary, Der, Diez Roux, Fitzpatrick, Hajat, Halaschek-Wiener, Harris, Hunt, Jagger, Jeon, Kaplan, Kimura, Lansdorp, Li, Maeda, Mangino, Nawrot, Nilsson, Nordfjall, Paolisso, Ren, Riabowol, Robertson, Roos, Staessen, Spector, Tang, Unryn, van der Harst, Woo, Xing, Yadegarfar, Park, Young, Kuh, von Zglinicki and Ben-Shlomo2014). Shorter telomeres have been associated with early death in the general population (Weischer et al., Reference Weischer, Bojesen, Cawthon, Freiberg, Tybjaerg-Hansen and Nordestgaard2012) although findings are inconsistent (Bojesen, Reference Bojesen2013). Telomere length has been more robustly associated with CHD, independently of traditional vascular risk factors (Haycock et al., Reference Haycock, Heydon, Kaptoge, Butterworth, Thompson and Willeit2014). It is not yet understood exactly why women differ from men in the development of CHD, however to date women have been underrepresented in cardiovascular trials, resulting in a bias towards factors which are relevant to disease aetiology in men (Fairweather, Reference Fairweather2014).
Current National Institute for Health and Clinical Excellence (NICE) guidelines recommend the use of the QRISK2 risk assessment tool to assess risk for the primary prevention of CVD in both men and women up to 84 years (National Institute for Health and Care Excellence, 2014). There has been some debate as to whether or not the addition of circulating inflammatory markers, such as CRP, should now be included in screening measures for cardiovascular risk (Pearson et al., Reference Pearson, Mensah, Alexander, Anderson, Cannon, Criqui, Fadl, Fortmann, Hong and Myers2003; Peters et al., Reference Peters, Visseren and Grobbee2013). The main uncertainty seems to be whether the modest increases in risk associated with higher inflammation can produce significant health benefits. This is understandable when considering that population studies have shown that CRP is a relatively moderate predictor of CVD risk, yielding an odds ratio of 1.5 when comparing the top baseline tertile with the bottom (Danesh et al., Reference Danesh, Wheeler, Hirschfield, Eda, Eiriksdottir, Rumley, Lowe, Pepys and Gudnason2004). In addition, when compared with traditional risk factors, such as smoking and total cholesterol, CRP only slightly improved their predictive value. Our study demonstrates that men with comorbid depressive symptoms and high inflammation constitute a clinically meaningful risk category. In light of these findings, it might be worth considering inflammation as a cardiovascular risk factor in depressed men. This could help identify patients who may benefit from targeted prophylactic intervention, improving screening efficacy and cardiovascular outcomes.
Study strengths and limitations
The strengths of our study include the prospective design, the presence of both women and men and the nationally representative nature of the ELSA cohort. Despite this, our study also has limitations. The first limitation of this study is that we measured depressive symptoms and inflammation at only one-time point at wave 2 (2004–2005) and did not investigate any change in levels at a later point. This design was chosen in order to maximise the number of participants with inflammation and depressive symptoms at baseline and to allow a longer follow-up period to capture mortality.
Nevertheless, we did not find an association of depressive symptoms and mortality in the absence of inflammation. In a recent study using the same cohort, which considered depressive symptoms across several years, a dose-response association was observed between persistence of depressive symptoms across time and mortality risk (White et al., Reference White, Zaninotto, Walters, Kivimaki, Demakakos, Biddulph, Kumari, De Oliveira, Gallacher and Batty2016). Similar to these findings, data from the Longitudinal Aging Study Amsterdam also found that transient depressive episodes did not predict mortality although chronic depression did (Geerlings et al., Reference Geerlings, Beekman, Deeg, Twisk and Van Tilburg2002). Secondly, we also did not control for medication use in our analysis, as this data was not available. Statins, for example, present anti-inflammatory effects (Antonopoulos et al., Reference Antonopoulos, Margaritis, Lee, Channon and Antoniades2012) and therefore medication use may have interfered with current findings. Thirdly, our study had a smaller proportion of female deaths compared to male deaths and therefore it is unclear whether our lack of association in women reflects an absence of an effect or is a result of insufficient power. The only closest previous study to date investigated combined depressive symptoms and inflammation in the development of CVD and was restricted to men. Finally, in our study depressive symptoms were measured by an 8 item self-reported questionnaire, rather than a diagnostic interview. Short scales such as this have previously been criticised for a lack of specificity. To address this, we defined depressive symptoms using a conservative cut-off of 4, which increases the measure's ability to discriminate between true and false positives.
In conclusion, we demonstrated that men with concurrent depressive symptoms and increased inflammation constitute a high-mortality risk phenotype. This risk is particularly high for cardiovascular-related death. These findings have clinical implications for the treatment and prevention of depression and inflammation in men. Subgroups of depressed individuals with comorbid inflammation may benefit from additional anti-inflammatory pharmacotherapy. Further research is needed to investigate whether combined interventions improve outcomes.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S003329171800209X
Acknowledgements
Livia A. Carvalho is funded by the MRC Immunopsychiatry Consortium (RG71546).
Conflict of interest
None.