Article contents
Effects of oxygen and nitrate on ammonium uptake kinetics and adenylate pools in Phalaris arundinacea L. and Glyceria maxima (Hartm.) Holmb
Published online by Cambridge University Press: 05 December 2011
Synopsis
We studied the effects of oxygen (aerated versus O2 depleted ∼0.5 mg 1−1 O2) and nitrate (none versus 10 μmol 1−1) on the ammonium uptake kinetics and adenylate pools in two wetland plants differing in their degree of flood tolerance (Phalaris arundinacea L. and Glyceria maxima (Hartm.) Holmb.). The study was performed as a random block design in a growth chamber. The 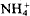 -uptake kinetics were estimated by using a computerised nonlinear parameter estimation procedure to fit the differential form of a modified Michaelis–Menten model to solution depletion curves. The uptake kinetics for
-uptake kinetics were estimated by using a computerised nonlinear parameter estimation procedure to fit the differential form of a modified Michaelis–Menten model to solution depletion curves. The uptake kinetics for 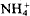 differed between the two species: Vmax was significantly higher for P. arundinacea (24.7 to 29.6 μmol h−1 g−1 root dry weight) than for G. maxima (4.6–10.3 μmol h−1 g−1 root dry weight). The
differed between the two species: Vmax was significantly higher for P. arundinacea (24.7 to 29.6 μmol h−1 g−1 root dry weight) than for G. maxima (4.6–10.3 μmol h−1 g−1 root dry weight). The 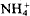 concentration at which uptake ceases (Cmin) was 0.2 to 0.5 μmol 1−1 for P. arundinacea and significant higher (1.1–2.7 μmol 1−1) for G. maxima.Km varied between 3.1 and 6.2 μmol 1−1 for P. arundinacea, and 1.6 and 3.0 μmol 1−1 for G. maxima. The different uptake kinetics of the two species reflect the different structure of their root systems: P. arundinacea has an extensive root system consisting of many thin roots whereas G. maxima has fewer but thicker roots. The uptake kinetics also suggest that P. arundinacea is adapted to growing at lower ambient
concentration at which uptake ceases (Cmin) was 0.2 to 0.5 μmol 1−1 for P. arundinacea and significant higher (1.1–2.7 μmol 1−1) for G. maxima.Km varied between 3.1 and 6.2 μmol 1−1 for P. arundinacea, and 1.6 and 3.0 μmol 1−1 for G. maxima. The different uptake kinetics of the two species reflect the different structure of their root systems: P. arundinacea has an extensive root system consisting of many thin roots whereas G. maxima has fewer but thicker roots. The uptake kinetics also suggest that P. arundinacea is adapted to growing at lower ambient 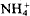 concentrations than G. maxima. Oxygen had no consistent effect on
concentrations than G. maxima. Oxygen had no consistent effect on 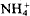 uptake kinetics. However, the plants that had
uptake kinetics. However, the plants that had 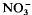 in the nutrient solution as well as
in the nutrient solution as well as 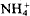 had slightly higher Vmax values and lower Cmin and Km values than those without
had slightly higher Vmax values and lower Cmin and Km values than those without 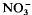 . Thus, both species were able to sustain their uptake characteristics at low external O2 concentrations, probably because of internal aeration through the air-space tissue of the plants. Nitrate deprivation also lowered the energy charge ratio and adenine nucleotide content in roots. The roots recovered quickly from
. Thus, both species were able to sustain their uptake characteristics at low external O2 concentrations, probably because of internal aeration through the air-space tissue of the plants. Nitrate deprivation also lowered the energy charge ratio and adenine nucleotide content in roots. The roots recovered quickly from 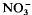 deprivation once
deprivation once 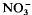 was resupplied. The stresses imposed by partially O2-depleted conditions and lack of nitrate were therefore relatively mild and reversible. It seems that the inherent aerenchyma development under aerated conditions in these species is sufficient to maintain adequate root oxygenation under partially O2-depleted conditions.
was resupplied. The stresses imposed by partially O2-depleted conditions and lack of nitrate were therefore relatively mild and reversible. It seems that the inherent aerenchyma development under aerated conditions in these species is sufficient to maintain adequate root oxygenation under partially O2-depleted conditions.
- Type
- Research Article
- Information
- Copyright
- Copyright © Royal Society of Edinburgh 1994
References
- 4
- Cited by




