Global context and consequences of stunting
In 2015, stunting affected approximately 159 million children under the age of 5 years worldwide and an important proportion of these children were in sub-Saharan Africa and South-central Asia( 1 ). It is projected that about 127 million children under 5 years will be stunted in 2025 if no meaningful preventive actions are taken( 2 ). In low- and middle-income countries, stunting is a huge public health burden that has consequences on long-term health( Reference Prendergast and Humphrey 3 ). In addition, linear growth faltering has multiple causal factors( 2 ), and is associated with poverty and hence a critical development indicator( Reference Kraemer 4 ).
In vulnerable populations, intra-uterine growth restriction is often associated with maternal undernutrition( Reference Dewey and Begum 5 ) and this may result in a vicious cycle of cross-generational stunting( Reference Victora, Adair and Fall 6 ). The incidence of stunting usually peaks around age 6–23 months as result of the transition from exclusive breastfeeding to introduction of complementary foods, which may be of poor nutritional quality( 7 , 8 ). In addition, infections can aggravate children's nutritional status and can contribute to stunting indirectly via the environmental enteric dysfunction mechanism( Reference Prendergast, Rukobo and Chasekwa 9 ).
Growth retardation, reduced work capacity and poor mental and social development can occur as a result of poor dietary intake during early childhood( Reference Souganidis 10 ). In addition, growth faltering is also affected by several non-dietary factors that are closely linked and multifaceted( 11 ). Nevertheless, the consequences of stunting may include delayed cognitive development and increased morbidity and mortality( Reference Victora, de Onis and Hallal 12 ).
Strategies to reduce stunting
Actions to address multiple forms of malnutrition are described in the Comprehensive Implementation Plan on Maternal, Infant and Young Child Nutrition which was endorsed by the World Health Assembly in 2012( 13 ). Other interventions include the WHO package of effective direct nutrition interventions( 8 ), the strategies for infant and young child feeding( 7 , 8 ) and nutrition-specific and -sensitive approaches as highlighted in the 2013 Lancet Maternal and Child Nutrition Series( Reference Ruel and Alderman 14 , Reference Bhutta, Das and Rizvi 15 ).
Improving maternal nutritional status coupled with appropriate infant and young child feeding during the critical first 1000 d (from conception to age 24 months) window can result in reduced morbidity and mortality, with notable benefits on growth and development for children( 7 ). Evidence-based, innovative and affordable interventions such as exclusive breastfeeding, appropriate introduction of complementary foods coupled with continued breastfeeding from age 6 to 23 months or beyond can help prevent growth failure( 8 , 16 , 17 ). This eventually may have a long-term impact on global health and development( Reference Bhutta, Das and Rizvi 15 , Reference Gruszfeld and Socha 18 – Reference Haddad, Hawkes and Achadi 20 ). To address growth faltering and break the intergenerational cycle of undernutrition a total lifecycle approach is necessary to fully address malnutrition. Improving pre-conceptual nutritional status contributes to the prevention of intra-uterine growth restriction, which may result in lower risk for low birth weight and lower risk of stunting( Reference Bhutta, Das and Rizvi 15 , Reference Nabarro 21 ).
Interventions targeting complementary feeding are usually focused on the age range of 6–23 months( 7 , Reference Bhutta, Das and Rizvi 15 ). This is the period of a high incidence of growth faltering, micronutrient deficiencies and infectious illnesses in developing countries. In low-income settings, consumption of plant-based complementary foods, which are usually deficient in key micronutrients (particularly iron, zinc and vitamin B6) often results in sub-optimum child growth and development( 7 , 8 ). Therefore, interventions that provide fortified complementary food supplements as ‘point of use’ or ‘home’ fortificants, or eaten alone as snacks have potential to improve both macronutrient and micronutrient intake( 22 , Reference Dewey 23 ). A number of complementary food supplements have been developed, which include fortified spreads( Reference Briend 24 ), water-dispersible or crushable micronutrient tablets( Reference Gross 25 ), micronutrient powders( Reference Wang, Chen and Wang 26 ) and small-quantity lipid-based nutrient supplements (SQ-LNS)( Reference Hess, Abbeddou and Jimenez 27 – Reference Adu-Afarwuah, Lartey and Brown 29 ).
Nevertheless, it is important to note that dietary interventions during the complementary feeding period may not be sufficient, and integrated multifaceted interventions, addressing the various underlying causes of child malnutrition, are needed. Timing of these interventions are important, as maternal undernutrition increases the risk for growth restriction in utero; the first 1000 d is now thought to be the critical period for intervening( Reference Ruel and Alderman 14 , Reference Bhutta, Das and Rizvi 15 ). This indicates the need for comprehensive nutrition action in vulnerable communities in order to achieve the World Health Assembly target of reducing by 40 % the prevalence of stunting in children under 5 years by 2025( 2 ).
Small-quantity lipid-based nutrient supplements as strategy to improve linear growth
Although micronutrient interventions have received much attention as a cost-effective and promising strategy to improve child health, the results of multiple micronutrient intervention studies have been inconclusive( Reference Souganidis 10 ). Subsequently, lipid-based nutrient supplements (LNS) usually in form of SQ-LNS were designed to provide energy, protein, macro-minerals and essential fatty acids, in addition to micronutrients( Reference Hess, Abbeddou and Jimenez 27 ).
The SQ-LNS are currently at the centre of interest of academic research as a cost-effective and affordable method to ensure that children's recommended nutrient intakes are met, and further to reduce anaemia and prevent stunting in children aged 6–23 months. A broad spectrum of LNS products has been developed over the past decades. Ready-to-use therapeutic foods (100 g; 2092 kJ (500 kcal)/serving) or large-quantity LNS products such as PlumpyNut® were developed to treat severe acute malnutrition( Reference Arimond, Zeilani and Jungjohann 30 ). Medium-quantity LNS or ready-to-use supplementary foods (40–50 g; 1046 kJ (250 kcal)/serving) are designed to provide more than half of daily energy requirements and are used in the nutritional management of severe acute malnutrition and moderate acute malnutrition( Reference Arimond, Zeilani and Jungjohann 30 ). Small-quantity LNS products (such as NutriButter®) are designed to supply a lower energy dose (20 g; 460–628 kJ (110–150 kcal)/serving) and 50 % of recommended nutrient intakes for micronutrients and essential fatty acids. SQ-LNS are more suitable as home fortificants, for longer duration use, and are used for prevention of undernutrition in more food secure situations to fill certain nutrient gaps in the diet( Reference Dewey and Arimond 31 ). SQ-LNS products are covered by CODEX CAC/GL 8–1991, and are classified by the World Health Assembly as home fortificants and they are excluded from the guidance on ending inappropriate marketing of foods for infants and young children( 16 ).
A daily ration of SQ-LNS (20 g sachet) provides energy (about 460–502 kJ (110–120 kcal), protein, essential fatty acids and approximately twenty-two micronutrients, including zinc( Reference Arimond, Zeilani and Jungjohann 30 ). SQ-LNS are cost effective compared with high-energy-dense products with similar formulations and are thus more affordable for low-income consumers( Reference Arimond, Zeilani and Jungjohann 30 ). SQ-LNS provide low energy to ensure that breast milk intake is not compromised( Reference Giugliani, Horta and Loret de Mola 32 ) and allows for higher intakes of local foods, including animal-source foods, fruit and vegetables( Reference Arimond, Zeilani and Jungjohann 30 ). Based on experiences from previous and ongoing studies researchers from the International Lipid-based Nutrient Supplements project (http://www.iLiNS.org) published an overview on key issues to be considered when developing SQ-LNS for the prevention of linear growth faltering( Reference Arimond, Zeilani and Jungjohann 30 ).
Acceptability of small-quantity lipid-based nutrient supplements
To achieve their intended benefits, SQ-LNS need to be acceptable to the target groups in terms of organoleptic properties and user-friendliness in household settings( Reference DePee 33 , Reference Ashorn, Alho and Arimond 34 ). Poor sensory attributes of SQ-LNS( Reference Arimond, Zeilani and Jungjohann 30 , Reference DePee 33 ) can lead to low adherence to the supplementation regimen for the test products. Short-term studies to assess acceptability of SQ-LNS were done among 6–12 months old children and their caregivers in South Africa( Reference Rothman, Berti and Smuts 35 ), infants and pregnant or lactating women in Ghana( Reference Adu-Afarwuah, Lartey and Zeilani 36 ), 9–15 months old children and their mothers in Burkina Faso( Reference Hess, Bado and Aaron 37 ), and 8–12 months old children and their caregivers in Malawi( Reference Phuka, Ashorn and Ashorn 38 ). Long-term acceptability was assessed in 6–18 months old children in Malawi( Reference Ashorn, Alho and Arimond 34 ).
Although acceptability of SQ-LNS is typically assessed via sensory evaluation based on the child's willingness to consume the test meal and mother's perceived acceptance of the supplement using hedonic scales( Reference Arimond, Zeilani and Jungjohann 30 ), different approaches were used across studies. In the Burkina Faso( Reference Hess, Bado and Aaron 37 ), Ghana( Reference Adu-Afarwuah, Lartey and Zeilani 36 ), Malawi( Reference Phuka, Ashorn and Ashorn 38 ) and South African( Reference Rothman, Berti and Smuts 35 ) studies, acceptability testing consisted of evaluation of a test meal (or meals) and a 2-week home trial. Acceptability was measured based on the amount of test meal consumed, the time in which the test meal was consumed, the mother's evaluation of the sensory attributes of SQ-LNS (using a hedonic scale), the mother's perception of the infant's acceptance, and ease of use at home. The results of these short-term studies showed that SQ-LNS were well accepted by children and their mothers. In one of the efficacy studies done in Malawi( Reference Ashorn, Alho and Arimond 34 ), acceptability of SQ-LNS was assessed over the 1-year intervention period. Acceptability was defined based on adherence to the feeding regimen and the mothers’ experiences of feeding SQ-LNS to their children (from age 6 to 18 months). Results of this study showed that acceptability was sustained over the 12-month period. Sustained acceptability and ease of use at the household level are crucial to have impact on child growth( Reference Ashorn, Alho and Arimond 34 ), and future studies should therefore assess the acceptability of long-term use of SQ-LNS in addition to evaluating sensory attributes( Reference Arimond, Zeilani and Jungjohann 30 ).
For SQ-LNS to have the desired nutritional impact, they should be consumed as per intended protocol. Using SQ-LNS as home fortificants should therefore not alter the taste of the usual complementary foods; for example they should not be too oily( Reference Adu-Afarwuah, Lartey and Zeilani 36 ). Consequently, the acceptability SQ-LNS can be influenced by the effect of specific ingredients on the organoleptic properties of the final product( Reference Arimond, Zeilani and Jungjohann 30 ). Peanut-based SQ-LNS was shown to be acceptable in African( Reference Adu-Afarwuah, Lartey and Zeilani 36 – Reference Paul, Muti and Chasekwa 41 ) and non-African studies( Reference Heidkamp, Stoltzfus and Fitzgerald 42 – Reference Mridha, Chaparro and Matias 44 ), while a soya-based SQ-LNS was shown to be acceptable in a study in South Africa( Reference Rothman, Berti and Smuts 35 ). Although overall acceptability of these SQ-LNS products were shown, there is still limited evidence on the mothers willingness to pay for SQ-LNS should they be commercially available( Reference Segrè, Winnard and Abrha 45 ).
Current evidence on efficacy of small-quantity lipid-based nutrient supplements on linear growth
There is a growing body of evidence on the efficacy of SQ-LNS on the prevention of linear growth faltering( Reference Arimond, Zeilani and Jungjohann 30 , 46 ). Although the present paper is not a generic systematic review, we employed a systematic approach, as described by Khan et al.( Reference Khan, Kunz and Kleijnen 47 ) to select relevant literature. A comprehensive literature search was conducted for studies reported in the English language on PubMed, Google Scholar and Cochrane Library. The search words and phrases used included the following: stunting, lipid nutrient supplements, complementary food supplements, linear growth faltering, home fortificants, supplementation, length for age z-score (LAZ), children 6–23 months old. These words and phrases were used either separately or in combination. The articles appearing in reference lists of identified papers were also used for secondary search. The aim was to assess literature on the efficacy of SQ-LNS in the prevention of growth faltering in children under 2 years old. A summary of the results of these studies is given in Table 1. This section presents results of efficacy studies for SQ-LNS used for infants (from age 6 to 12 months) and for children (from age 12 to 18 months) and finally, prenatally, during pregnancy and for 6 months postnatally.
Table 1. Efficacy trials investigating the impact of the provision of small-quantity lipid-based nutrient supplements (SQ-LNS) on linear growth for infants/young children
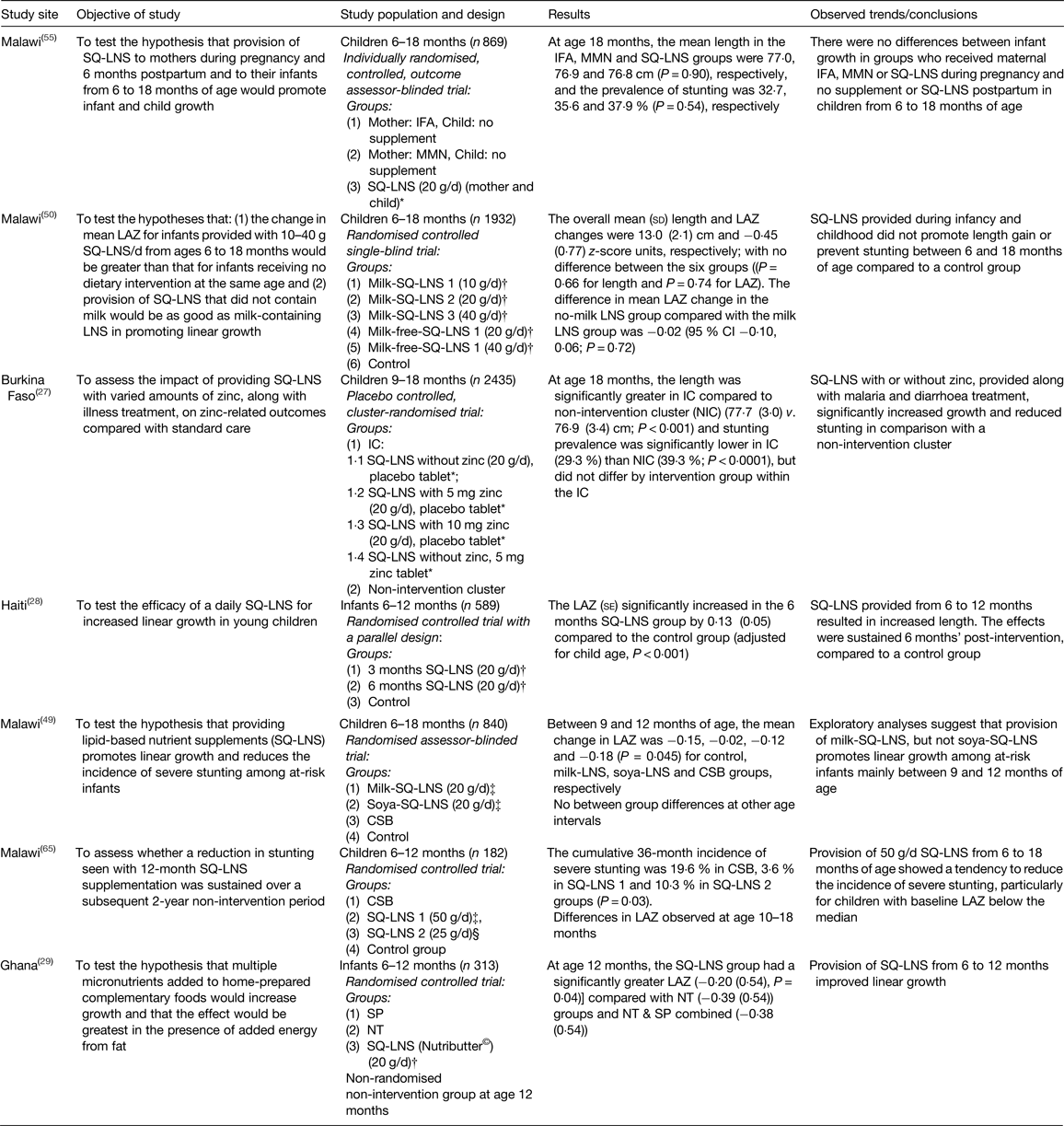
IFA, iron folic acid capsules; MMN, micronutrient capsules; LAZ, length for age z-scores; IC, intervention cluster; CSB, maize–soya blend; SP, Sprinkles powder; NT, crushable Nutritabs.
* SQ-LNS, International Lipid-Based Nutrient Supplements (iLiNS) (California Davis, USA).
† SQ-LNS, Nutributter® (Nutriset SA, Malaunay, France).
‡ SQ-LNS, Project Peanut Butter (Blantyre, Malawi), The Nutributter® and iLiNS formulations for infants/young children, pregnant and lactating women compared to WHO/FAO( 66 ) Recommended Nutrient Intakes were presented in detail by Arimond et al.( Reference Arimond, Zeilani and Jungjohann 30 ).
Small-quantity lipid-based nutrient supplements used for infants (age 6–12 months) and children (age 12–18 months)
Provision of SQ-LNS showed positive effects on linear growth in infants from 6 to 12 months in Haiti and Ghana( Reference Iannotti, Dulience and Green 28 , Reference Adu-Afarwuah, Lartey and Brown 29 ), and in children from 9 to 18 months in Burkina Faso, regardless of whether SQ-LNS contained zinc( Reference Hess, Abbeddou and Jimenez 27 ). In Malawi, providing SQ-LNS to children from 6 to 18 months showed limited effects. In the first study, provision of SQ-LNS showed a tendency to reduce the incidence of severe stunting, particularly in children who already showed growth faltering (LAZ < median) at baseline( Reference Phuka, Maleta and Thakwalakwa 48 ). Results of the second study suggest that provision of milk SQ-LNS, but not soya SQ-LNS, promotes linear growth between 9 and 12 months, but not thereafter( Reference Mangani, Maleta and Phuka 49 ). On the contrary, another study in Malawi failed to show any effect of SQ-LNS on linear growth in infants from 6 to 18 months regardless of whether the SQ-LNS contained milk( Reference Maleta, Phuka and Alho 50 ). For the studies reviewed, results on the impact of providing SQ-LNS for infants (6–12 months old) and children (12–18 months old) on preventing stunting is still inconclusive.
Provision of small-quantity lipid-based nutrient supplements to pregnant and lactating mothers and infants from age 6 months old
SQ-LNS have also been designed to fortify maternal diets with micronutrients and essential fatty acids( Reference Arimond, Zeilani and Jungjohann 30 ).
However, trials that have investigated the effect of providing SQ-LNS to mothers during pregnancy and for 6 months postpartum, and subsequently to the child from age 6 months onwards on birth outcomes and stunting have produced mixed results( Reference Adu-Afarwuah, Lartey and Okronipa 51 – Reference Ashorn, Alho and Ashorn 55 ). Efficacy trials in Ghana showed that providing SQ-LNS for 6 months prenatally and a further 6 months postpartum to mothers and to children from age 6 to 18 months had positive effects on birth outcomes and linear growth in children( Reference Adu-Afarwuah, Lartey and Okronipa 51 , Reference Adu-Afarwuah, Lartey and Okronipa 54 ). In Bangladesh, SQ-LNS given for 6 months prenatally and a further 6 months postpartum to mothers and to children from age 6 to 18 months improved birth outcomes( Reference Mridha, Matias and Chaparro 52 ). However, in Gambia provision of SQ-LNS prenatally did not show significant benefits on preventing intra-uterine growth restriction which is associated with childhood stunting( Reference Johnson, Darboe and Sosseh 53 ). The lack of intervention effect was also observed in the Malawi trial( Reference Ashorn, Alho and Ashorn 55 ).
Differences in duration of trials, composition and dosage of SQ-LNS, and baseline demographics and nutritional status of participants make it difficult to directly compare studies. During a recent technical meeting to review evidence and pragmatic issues on provision of SQ-LNS as a preventative strategy for undernutrition, it was recommended that contextual factors and study design should be considered in the implementation of results of SQ-LNS trials( 46 ). Nevertheless, further research is required to understand the potential growth-promoting effect of SQ-LNS and certain ingredients in SQ-LNS, such as milk powder and essential fatty acids( Reference Dewey and Arimond 31 ). At pragmatic level as part of integrated nutrition interventions, behaviour change communication may be necessary to ensure appropriate utilisation of SQ-LNS and subsequent impact on linear growth( Reference Paul, Muti and Chasekwa 41 , Reference Kodish, Aburto and Hambayi 56 ).
Factors affecting efficacy of small-quantity lipid-based nutrient supplements on linear growth
Although efficacy trials are carried out under ideal and controlled conditions( Reference Gartlehner, Hansen and Nissman 57 ), the differences in the study design and settings across different trials may partly explain the mixed results on the impact of SQ-LNS on linear growth. Therefore, the interpretation of results from efficacy trials depends on the study population, setting and design( 46 , Reference Gartlehner, Hansen and Nissman 57 , Reference Singal, Higgins and Waljee 58 ). This is particularly important for complementary feeding interventions that are expected to have impact for children 6–23 months old. Complementary feeding interventions usually report small to medium effects on child growth as there are many factors that influence child growth besides dietary intake( Reference Dewey and Adu-Afarwuah 59 ). The efficacy of complementary food supplements such as SQ-LNS on child growth is influenced by factors that include but are not limited to the following: the characteristics of the target group (i.e. baseline nutritional status, age, withdrawal rates); the study setting (socioeconomic status, infections) and design (adherence calculation, control group, duration of intervention)( Reference Dewey and Adu-Afarwuah 59 ). These factors can affect the internal and subsequent external validity of results of efficacy trials, and this makes it difficult to assess the impact of the intervention in the absence of the baseline prevalence of stunting. The internal validity of trial results can also be a factor of robust inclusion criteria, randomisation and blinding, baseline nutritional and socioeconomic status of participants and data quality management, adherence monitoring and duration of the intervention( Reference Arimond, Zeilani and Jungjohann 30 , 46 ).
A study in Malawi showed that the provision of milk-SQ-LNS, but not soya-SQ-LNS promotes linear growth among at-risk infants aged between 9 and 12 months, but not from 12 to 18 months( Reference Mangani, Maleta and Phuka 49 ); overall evidence on an intervention effect of provision of SQ-LNS on stunting prevalence was inconclusive. Mangani et al.( Reference Mangani, Maleta and Phuka 49 ) reasoned that the observed prevalence of stunting across study groups in the Malawi study could have been influenced by the high incidence and prevalence of morbidity and associated environmental enteropathy( Reference Prendergast, Rukobo and Chasekwa 9 ) or poor prenatal and maternal nutritional status( Reference Christian, Lee and Donahue Angel 60 ). In addition, the researchers hypothesised that the constant dose of 20 g/d may not be sufficient as the children get older and have increased nutrient requirements. Results from a recent trial in Malawi also reported no effect of SQ-LNS supplementation prenatal and postpartum to women and their children( Reference Ashorn, Alho and Ashorn 55 ). The researchers attributed the lack of effect on stunting on some technical difficulties in supply of SQ-LNS to participants, high attrition rate, low-energy dose of the SQ-LNS regimen, low compliance to intervention protocol and low adherence for SQ-LNS for children (77·1 %). In addition, the results also suggested possible effect of underlying infections that may indirectly restrict linear growth( Reference Prendergast, Rukobo and Chasekwa 9 ).
Another trial in Malawi showed no effect of SQ-LNS on linear growth in children aged 6–18 months( Reference Maleta, Phuka and Alho 50 ). This finding could have been influenced by high rate of attrition or mobility, technical difficulties in supply of SQ-LNS to participants, inability to verify self-reported supplement consumption (self-reported adherence of 92·9 % v. reported consumption rate of 71·6 %). The lack of standardised methods of calculating adherence makes it difficult to make comparisons across studies.
Another probable reason why SQ-LNS efficacy trials report low impact on linear growth could be that the usual 20 g/d may not be sufficient for older children as their nutrient requirements do increase with age( Reference Mangani, Maleta and Phuka 49 ). There are also indications that LAZ may not be an appropriate indicator to assess changes in length over time compared with height-for-age difference (child's height compared to reference height, expressed in centimetres)( Reference Leroy, Ruel and Habicht 61 ). There may be need to investigate the use of height-for-age difference v. LAZ in assessing the intervention effects of SQ-LNS on linear growth( Reference Leroy, Ruel and Habicht 61 ).
The factors discussed earlier highlight the importance of appropriate study design and data quality on the interpretation of trial results to ascertain impact of SQ-LNS on linear growth.
Therefore, future studies should be designed to accurately assess total nutrient intake, utilise reliable indicators of estimating actual consumption of SQ-LNS, maintain acceptability of SQ-LNS and based on comprehensive situation analysis in the context of target communities( Reference Kodish, Rah and Kraemer 62 ).
In summary, there is inconclusive evidence on the efficacy of SQ-LNS supplementation on improving linear growth in infants and children and more trials are required to provide insight into this area. Therefore, there is need for pragmatic trials to assess the impact of integrating SQ-LNS with already existing interventions targeted at girls and women of child bearing age, such as availability of safe drinking-water, basic sanitation and hygiene, malaria and infection control in different contexts. Behaviour change communication may be necessary to ensure appropriate utilisation of SQ-LNS and associated impact on linear growth( Reference Paul, Muti and Chasekwa 41 , Reference Kodish, Aburto and Hambayi 56 ).
Recommendations for future research
There is evidence that linear growth faltering affects children beyond the first 1000 d in low-and middle-income countries( Reference Leroy, Ruel and Habicht 63 ) and the implications of this on the timing for interventions to reduce stunting still needs to be explored. Therefore, there will be need for SQ-LNS trials with longer follow-up to assess if the benefits can be maintained beyond age 2 years. These studies can also investigate the use of height-for-age difference v. LAZ to assess the intervention effects of SQ-LNS interventions on linear growth( Reference Leroy, Ruel and Habicht 61 ). Trials in Malawi have shown that there is need to explore the impact of the enteropathy mechanism on child growth in low-income settings( Reference Maleta, Phuka and Alho 50 , Reference Ashorn, Alho and Ashorn 55 ). Iannotti et al.( Reference Iannotti, Dulience and Green 28 ) highlighted the need for SQ-LNS effectiveness studies. The contribution of SQ-LNS to the prevention of growth faltering is still unclear and more research needs to be done to produce more conclusive results. There is need for standardised methods to assess adherence in community-based supplementation trials( Reference Abbeddou, Hess and Yakes Jimenez 64 ). This will enable accurate accountability of SQ-LNS utilisation and enable possible interpretation of study outcomes across studies.
Future studies can also explore how to maintain optimum dosage (>20 g/d) for children as they get older( Reference Mangani, Maleta and Phuka 49 ). Accurately recording of morbidities that commonly occur during this critical period of development (age 6–23 months) should further improve the interpretation of infant growth and development outcomes. Overall there is great need for providing SQ-LNS as part of integrated and comprehensive nutrition interventions and to ascertain the cost and comparative cost-effectiveness of different integrated strategies( Reference Dewey and Arimond 31 ) to prevent stunting in low-income settings.
Conclusions
The results of the studies reviewed showed inconclusive evidence on the efficacy of SQ-LNS to improve linear growth in children under 2 years. To be effective there is need to critically consider contextual factors and to integrate the provision of SQ-LNS with existing interventions aimed at addressing growth faltering in low-income settings.
Acknowledgements
The authors are grateful to the organisers of the 7th Africa Nutritional Epidemiological Conference (ANEC VII) 2016 for the invitation to present the present paper. We thank Sheila Gautier and colleagues from DSM for organising the Lipid Nutrition – New Insights Symposium at ANEC VII. We thank Jennifer Osei-Ngounda for the proofreading.
Financial Support
None.
Conflict of Interest
None.
Authorship
T. M. conducted the literature search and drafted the paper of which the co-authors contributed in many respects. M. F., H. S. K. and C. M. S. were involved in the conceptualisation, provided their broad knowledge and review of the paper. All authors read and approved the final manuscript. All authors had final approval of the submitted version.






