Introduction
Problematic substance use in the Middle East
Substance misuse exists in the Middle Eastern countries as well as in western societies. In the Middle Eastern countries substance misuse is complicated by the additional stigma of cultural and religious barriers. The most commonly used illicit drugs in the Middle East are opium, stimulants and marijuana supplied through Afghanistan and in more recent years, through Pakistan, Mexico and Thailand (Kenneth, Reference Kenneth2014). Saudi Arabia is the largest consumer of stimulants in the Middle East (Sloan, Reference Sloan2014). Jordan and Lebanon have smaller but still evident drug problems (Jordan, 2014). In Iran, drug ‘addiction’ is no longer punishable by law. However, ‘abuse’ of illicit substance still has legal consequences and can result in fines, lashings or a death sentence (~500 per year) (Adib, Reference Adib2014).
Over recent years, there has been a considerable increase in the number of reported substance misuse cases in the United Arab Emirates (World Drug Report, 2014). The Global Health Burden of Disease Study 2010 found that drug misuse, excluding tobacco and alcohol, increased by 526% from 1990 to 2010; although the exact number of drug users was not available (WHO, 2014). The report excluded tourists, but included expatriate residents who make up 85% of the UAE population. This time period corresponds to the economic and social changes experienced in the country as it transformed from seven sheikdoms in 1971 to the United Arab Emirates with its oil related wealth. UAE nationals have rapidly transcended from a nomadic, isolated existence to a relatively luxurious lifestyle.
Alcohol use is also of concern. Although alcohol use is strictly forbidden in the United Arab Emirates, a report by the World Health Organisation (WHO) found that its use in this part of the world was very high at almost twice the global average per year putting individuals in the highest health risk categories (WHO, 2015).
Seeking treatment for addiction has always been challenging in Islamic societies and an understanding of the alcohol and drug culture within this region is of great importance for successful prevention programmes, harm reduction strategies and treatment interventions (Abou-Saleh, Reference Abou-Saleh2006). In 2011, attitudes and beliefs of primary care physicians in Abu Dhabi towards the treatment of substance misuse related problems were surveyed (Marzouqi et al., Reference Marzouqi, Matheson, Bond, AlGhaferi and El Kashef2011). Results showed that only 8.7% of physicians currently treated patients with substance misuse disorders and most of the physicians (93.2%) did not have any specialised training. The survey also found that 66% of physicians were interested in obtaining further training in screening and management of patients with substance misuse-related problems.
The development of drug and alcohol screening in primary care
The use of the primary care infrastructure to identify individuals with problematic alcohol use was identified by the WHO in the 1980s. This led to the development of the AUDIT screening tool and the evaluation and implementation of brief interventions (BIs) for those using alcohol (or other illicit substances) at a harmful level (WHO, 2003a). However, subsequently, the WHO recognised that for some cultures, a tool that combined alcohol screening with other substances was required. This led to the development of the alcohol, smoking and substance involvement screening test (ASSIST) which has subsequently been evaluated across the world including Australia, Brazil, Ireland, India, Israel, the Palestinian Territories, Puerto Rico, the United Kingdom and Zimbabwe. Guidelines on the ASSIST and delivery of a subsequent BI for harmful or hazardous substance use are available through the WHO (2003b). This combined screening, BI and referral for treatment is known as the SBIRT programme and its use was extended to the developing world in 2002 with the publication of SBIRT guidelines (SBIRT, 2003). SBIRT has been widely tested and a body of evidence confirms its effectiveness and supports its use as an early intervention (Bernstein et al., Reference Bernstein, Bernstein, Tassiopoulos, Heeren, Levenson and Hingson2005; Babor et al., Reference Babor, McRee, Kassebaum, Grimaldi, Ahmed and Bray2007; Young et al., Reference Young, Stevens, Galipeau, Garrity and Singh2012). It has three steps (i) Screening (using ASSIST or AUDIT or DAST) to assess the severity of substance misuse and identify the level of treatment needed. (ii) Delivery of a BI to patients at risk to inform the patient of the problem and focus on behavioural change, (iii) Referral to specialist treatment for those severely affected by substance use.
This study aimed to determine (i) whether training primary care physicians in Abu Dhabi on the SBIRT model increased the identification of patients using drugs or alcohol at a harmful, hazardous or dependent level and (ii) whether SBIRT training improved physicians’ knowledge, attitudes and beliefs in self-efficacy for patients with substance misuse.
Methods
Study design
This was a non-randomised, controlled intervention trial comparing intervention and control clinics in terms of the reported prevalence of patients with problematic substance use. For the intervention group, quantitative patient data (ASSIST and a demographics questionnaire), quantitative physician data (questionnaires to assess attitudes and willingness to undertake SBIRT) and qualitative physician data via interviews were collected. In addition, the study included practice level quantitative data on all patient diagnoses and prevalence of drug-related conditions. This mixed method approach gave both breadth and depth to the evaluation. All strands of the study ran concurrently from December 2013 to October 2014. Qualitative findings are presented elsewhere (Pflanz-Sinclair et al., Reference Pflanz-Sinclair, Bond, Lee, El Kashef, Almarzouqi, Batieha and AlGhaferi2017). The study was approved by the National Rehabilitation Centre Ethical Review Committee. See Figure 1 for study flowchart.
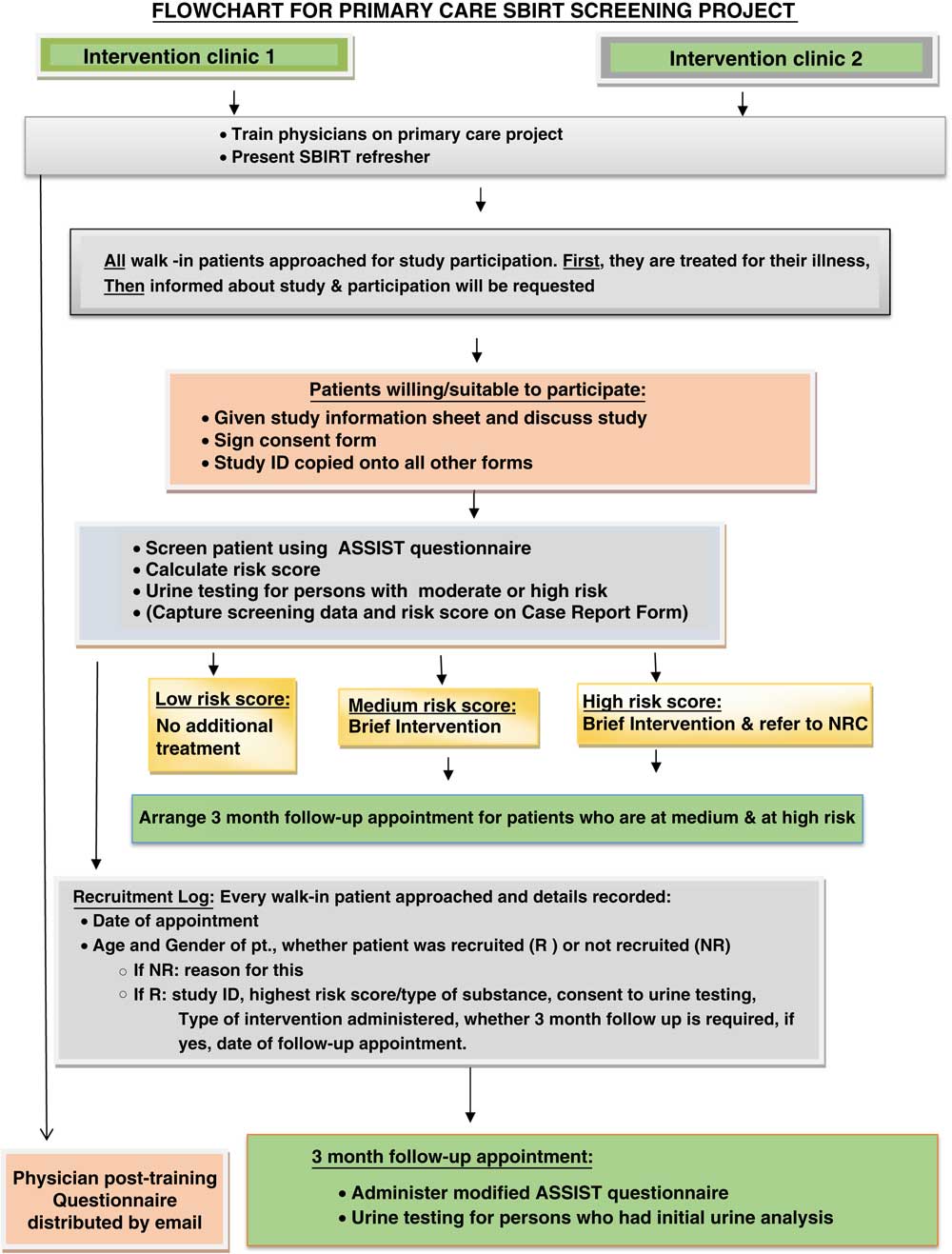
Figure 1 Flowchart for Primary Care SBIRT screening project. CES=cauda equina syndrome
Sample size
A sample size of 900 patients per arm was calculated to give 90% power at the two-sided 5% significance level to detect an absolute 5% difference (9% in control arm and 14% in intervention arm) in the reported prevalence of patients identified as using drugs and/or alcohol. As there were four clinics in total (two in the intervention arm and two in the control), each intervention clinic therefore aimed to recruit 450 patients.
Setting and participants
Two primary care centres in Abu Dhabi were selected as intervention sites by the Ambulatory Healthcare Services, according to patient demographics and social status, and willingness of clinic managers to participate in the study. All primary care physicians from these sites were invited to participate in the project. Matched control sites were then selected, (matched on patient demography) and prioritising geographical closeness. All sites were considered to have a large number of patients with substance use problems.
All 21 primary care physicians from the two intervention sites received an electronic invitation letter from the research team with a description of the project, its purpose and details of the training event. Physicians were invited to attend a two-day training event on SBIRT implementation and the data collection requirements for the trial.
Patient inclusion criteria: All UAE nationals aged ⩾18 years, willing to consent, who attended one of the intervention clinics as a walk in patient (ie, not scheduled appointments) during the recruitment period. Exclusion criteria were non-UAE nationals as they do not have access to the same state funded treatment facilities. A study poster was displayed in the waiting areas of the intervention clinics to inform patients that they might be asked to participate.
Data collection tools
Patient data collection consisted of the ASSIST screening tool and a demographics questionnaire. The ASSIST screened for use of tobacco, alcohol, cannabis, cocaine, amphetamine-type stimulants, inhalants, sedatives, hallucinogens, opiates and ‘other’ drug use. Completion of the ASSIST takes 7–10 min and the resultant risk scores are used to provide feedback to patients about their substance misuse. The score is grouped into ‘low’, ‘moderate’ or ‘high’ and determines the level of intervention required, that is ‘treatment as usual’, ‘brief intervention’ or ‘brief intervention plus referral to specialist treatment’. Although data was collected on tobacco, this was not a primary focus of the study as it is a legal substance with a different care pathway. The demographics questionnaire included age, gender, income, employment, marital status.
Primary care physician data collection used a structured questionnaire to assess the knowledge, attitudes and belief in self-efficacy (ie, whether they think they could deliver an effective intervention). The questionnaire was based on the Alcohol and Alcohol Problems Perception Questionnaire (AAPPQ), the Drug Problems Perceptions Questionnaire (DDPPQ), and the Aberdeen University General Practitioner attitude questionnaire (Matheson et al., Reference Matheson, Pitcairn, Bond and Ryan2003). These scales were shortened and modified by the research team for cultural relevance. The questionnaire was divided into three sections: (A) attitudes (B) physician’s practice and (C) the rates of screening and BI in daily practice. Sections A and C used a five-point Likert scale. Section B comprised fixed choice questions.
SBIRT training (the intervention)
Intervention clinic physicians were trained in SBIRT, that is, to screen patients using the ASSIST, to deliver a BI to those patients with moderate risk of harm and to refer patients with probable dependence to the specialist treatment centre. The training used the standard WHO methodology and modules from the Treatnet Training Package (2015). The SBIRT module which was used in training was Module 1 from volume A, screening and BI using the ASSIST. The training was delivered over two days by Thomas Babor, a world expert in SBIRT and included both didactic, and role-play. Before the start of patient recruitment, refresher training was held at each intervention clinic, delivered by two of the research team. It took the form of a 2-h meeting. A summary of SBIRT and the use of the ASSIST tool were explained, followed by a short explanation of the study protocol and a summary of the overall project procedures.
Data collection and management
The study was explained to patients by the physician after their consultation for the presenting condition. Physicians were asked to include all patients that met the inclusion criteria (ie, no selection or exclusion for any other reason than specified in the exclusion criteria). Patients were given a patient information sheet and invited to discuss the project with the physician. If willing to proceed, consent was obtained then the physician administered the ASSIST and demographics questionnaire to the patient. Any patient who scored a moderate or high risk score in the ASSIST for any substance except tobacco received a BI and was asked to return for a follow-up appointment three months later. Additionally any patient scoring ‘high’ was referred to a specialist centre for treatment. Those using tobacco were referred to a smoking cessation clinic and no further data was collected on them.
The physician questionnaire was distributed to intervention physicians at three separate time points: (i) handed out immediately before the start of SBIRT training, (ii) handed out immediately after training and (iii) eight months after SBIRT implementation. Physicians from the control clinics were only informed about the project by email after implementation, and that their clinic had been identified by the Health Authority as a control site. The intention was to reduce the Hawthorne Effect of changing behaviour regarding detection of SUD. Physicians were asked to complete and return the questionnaire electronically at the third time point only. Reminder emails were sent.
Quantitative data was entered in SPSS 22 for analysis. From the physician questionnaire, each level of the five-point Likert scale of Section A (Attitudes) and Section C (Screening and BI) had a numeric value attached, and negatively phrased questions were recoded into a new variable so that all codes and attached values were in the same positive direction. The numeric values were then summed so that each physician had a score representing their attitudes and a separate score for their involvement in screening and BI. A 10% data check was performed by a second researcher for quality assurance.
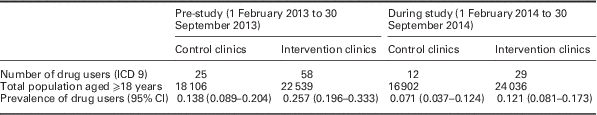
Routine data, collected on the electronic patient management system (Cerner), was used to determine the percentage of attendances associated with a drug-related problem (identified using ICD 9 codes) for each of the four clinics for two separate periods, 1 February 2013 to 30 September 2013, (before recruitment), and 1 February 2014 to 30 September 2014 (during recruitment). Multiple attendances for individual patients were identified and only one attendance was included in the data to avoid inflating the estimates of the background prevalence of problem drug use. Note alcohol-related conditions were excluded from the analysis of routine data due to challenges in identifying all potential alcohol-related problems. Patients recruited and screened as part of the intervention study were not recorded in this routine patient management system to ensure there was no double counting.
Statistical analysis
Descriptive statistics included n (%) for categorical variables such as demographic factors and mean (SD) for continuous variables such as physician attitude scores. Physician scores were compared between time points using the paired t-test. The prevalence of drug misuse from routine data was compared between intervention and control clinics using the chi χ 2 test. Where numbers warranted, the association between each of the demographic factors and substance misuse categories were examined using the χ 2 test or if there were small numbers in some groups rendering the χ 2 test invalid, then categories were collapsed and the test re-run. If this needed to be repeated resulting in a 2 by 2 table, then Fisher’s exact test was performed.
Results
Recruitment and demographics
A total of 17 primary care physicians (81% of those eligible) attended the main SBIRT training event and completed a questionnaire before the start of the training (first time point). Of those, 15 completed the questionnaire at the end of training (two had had to leave early). In all, 11 of the original physicians completed the questionnaire at time point 3, post implementation. Only five of the 21 control group physicians returned questionnaires (23.8%) despite reminders. Due to potential high risk of responder bias, it was decide not to use these questionnaires in the planned comparative analysis and to proceed with a before and after analysis for the intervention group only.
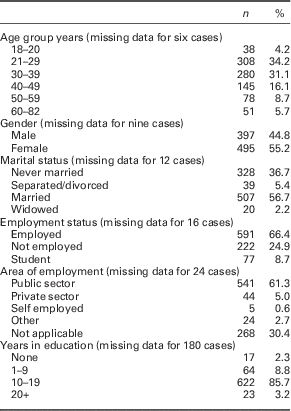
A total of 906 patients was recruited and completed the demographic questionnaire. The mean (SD) age was 35.6 years (12.6), range 18–82 years. The age group with the highest number of participants recruited was 20–29 years (see Table 1).
Table 1 Demographics of screened participants (n=906)
Physician attitudes and willingness to deliver SBIRT
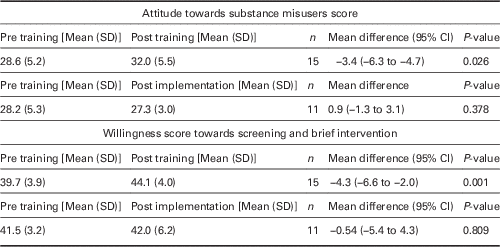
For intervention physicians, there was a significant increase in the attitude scores between pre training and post training (P=0.026). There was no significant change in the pre-training and post implementation attitude scores (P=0.378).
There was a significant increase in the screening and BI scores between pre training and post training (P=0.001). However, there was no significant change in the pre-training and post implementation screening and BI scores (P=0.809) (see Table 2).
Table 2 Physician attitudes and willingness towards substance misusers and SBIRT
Substances used and association with demographic factors
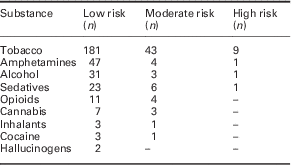
Tobacco was the most frequently used substance followed by alcohol, then amphetamines (see Table 3).
Table 3 Number of patients scoring low, moderate or high on alcohol, smoking and substance involvement screening test (ASSIST) by substance
Supplementary Material Tables 1–3 summarise the associations of demographic factors with each of amphetamine use, sedative use and the use of any drug/alcohol. Amphetamine users generally showed a higher number of years of education than non-users (P=0.049). A significant association between sedative use and age group was found (P=0.003) with the age group of 40+ having the highest number of sedative users. Significantly more males than females were found to use any drug/alcohol (P=0.016).
BI and referral for those with moderate and high ASSIST scores
There were 25 (2.3%) moderate or high scores for risk of substance misuse, excluding tobacco smokers. Four of these participants were multi drug users resulting in a total number of 21 participants that should have received a BI and returned for a follow-up appointment. Of these, the majority screened medium or high risk for sedatives (n=7) followed by amphetamines (n=5) and opioids (n=4). Three participants had a high risk score (one for amphetamine, one for alcohol and one for sedatives) and were referred for specialist treatment. No patients (moderate or high scoring) attended follow-up treatment.
Of the 21 participants with a moderate or high ASSIST score, 12 (57.1%) were male, 13 (61.9%) were in the 30–39-year age band, four (19%) were aged 20–29 years and four (19%) were in the 40–49 year age band. In all, 12 (57.1%) of the 21 were in employment, 11 of them in the public sector, 11 (52.4%) were never married.
Clinic level prevalence of recorded patients with problematic drug use
In the pre-study period, the prevalence of people with problematic drug use recorded in the intervention clinics was significantly higher than in the control clinics (0.257% versus 0.138%, P=0.008). During the study period, there was no significant difference in the recorded prevalence of drug users in the intervention compared with the control clinics (0.121% versus 0.071%, P=0.118).
Examination of the change from pre-study to during study period showed some evidence of a fall in prevalence of drug users among the control clinics (0.138% pre versus 0.071% during, P=0.054). In the intervention clinics, a significant decrease in prevalence of drug users was seen (0.257% pre versus 0.121% during, P=0.001) (see Table 4).
Table 4 Background prevalence of drug use from routine clinic data
When the 21 patients using substances were identified in our SBIRT intervention study as having a moderate or high risk on the ASSIST were added to the 29 identified drug users across the intervention clinics data, the prevalence (95% CI) of identified drug use in the intervention clinics increased significantly to 0.208 (0.154–0.274, P=0.018). This adjusted figure was significantly higher than the prevalence of drug users in the control clinics (P<0.001).
Discussion
Key findings
Of the 906 people screened in the two intervention clinics, 21 had a moderate or high score for any substance (excluding smoking) and required a BI. Of these, the majority screened medium or high risk for sedatives (n=7) followed by amphetamines (n=5) and opioids (n=4). Smoking was not a focus of the current project, but 52 participants had a moderate or high ASSIST score for smoking tobacco. SBIRT had a positive effect in detecting drug-related conditions at a clinic population level.
Analysis of those screening medium or high risk (excluding tobacco) by demographics found a few statistically significant associations. There was a significantly higher proportion of men using alcohol and significantly more in the 18–29-year age group screened positive for alcohol and for sedatives. However, due to the small numbers of participants taking one or more of the listed drugs, there was limited statistical power to identify significant associations with demographic variables and hence a multivariate analysis was not justified.
Strengths and limitations
Strengths of the study were that it recruited to target and the total sample size was large ensuring generalisability and precise estimates. A further strength was that since it was the first study of its kind in primary care in the Middle East, there was a lot that could be learned for future research and service implementation. A final strength, although reported separately (Pflanz-Sinclair et al., Reference Pflanz-Sinclair, Bond, Lee, El Kashef, Almarzouqi, Batieha and AlGhaferi2017), was the concurrent qualitative process evaluation which allowed considerable light to be shed on the reason behind the lack of apparent effect of screening (Jick, Reference Jick2006).
A weakness of the study was that it did not use a randomised control study design, as had been originally planned, due to the complexities of working with local health authorities and local regulatory bodies in the United Arab Emirates where such research in practice is still a new concept. This is particularly important as it indicates a need to develop the disciplines of Public Health and Health Services Research in this part of the world. Intervention clinics had higher levels of substance use at baseline which was why they were selected by the Health Authority for inclusion and probably introduced some bias into the study. This was despite control clinics also supposedly having suspected high level of substance use. The control clinics were not invited to participate at baseline and were only involved after screening was complete. The advantage of this was that background routine prevalence data should not have been contaminated. However, the disadvantage was that it was challenging to encourage control clinic physicians to subsequently complete a questionnaire. A further weakness of the design was that only UAE nationals were included in the screening programme. The cultural differences and reticence in revealing substance use may be more pronounced in the UAE national population than in the wider population. Although we examined the associations of demographic factors with drug use categories, due to small numbers we were underpowered to detect statistical significance.
The use of a sample size calculation was appropriate (despite not actually recruiting patients in the control group) for the comparison of two independent groups (intervention clinics versus control clinics) because the outcome was the between group difference in the prevalence of problematic substance use rather than simply the impact of the SBIRT training within the intervention practices.
A final limitation was that none of those identified as at moderate or high risk returned for follow-up despite being contacted by practices. In retrospect, a three-month follow-up period may have been too long for this group in this cultural context and may have contributed to patients not attending. However, this is a finding that can be used to strengthen future implementation. Ideally these patients should have been contacted by the research team to determine why they did not return for a follow-up visit but this was not possible in the time available.
Screening using SBIRT
The primary objective of the study was to determine whether training primary care physicians in Abu Dhabi on the SBIRT model increased the number of patients that were identified with substance use problems. According to the clinical prevalence and screening data, this was the case when those identified through the screening intervention (who were not recorded on the management system) were added to the total patients with ICD codes relating to problem drug use on the patient management system.
A few people screened positive for substance use and, as a result, a few received a BI. Data from qualitative interviews suggested that physicians believed there was a bigger problem in their patient group than screening indicated. It was suggested that people were reticent about admitting substance use for fear of involvement of the police (Pflanz-Sinclair et al., Reference Pflanz-Sinclair, Bond, Lee, El Kashef, Almarzouqi, Batieha and AlGhaferi2017).
In total, just 21 individuals received a BI from 906 people screened, a rate of 1.8%. Comparison with the international literature has not been possible as a comparable model of using the ASSIST in a general primary care population has not, to our knowledge, been identified.
Of the 906 patients screened, three should have been referred for specialist treatment based on their ASSIST score. These patients were not followed up beyond referral because their care was then out with primary care services. It became clear from interview data, presented in a linked qualitative paper that referral to the specialist service was not normal practice for primary care physicians who might refer elsewhere (Planz et al., submitted). As this care pathway was new and physicians themselves did not feel they knew much about the treatment that would be provided by the specialist service, there may have been some difficulty in communicating to patients the importance of follow-up appointment attendance. In a study by Madras, follow-up was conducted either by phone or in person and attendance rates varied considerably, illustrating that more research needs to be done to understand and improve follow-up after SBIRT (Madras et al., Reference Madras, Compton, Avula, Stegbauer, Stein and Clark2008).
Physician attitudes
Physicians generally demonstrated a positive attitude towards patients with substance use problems s and their role in managing problematic substance use through the application of the SBIRT programme. Training proved to be important because the attitude scores immediately post training were significantly improved compared with pre-training scores, despite the relatively small numbers of physicians trained. This indicates training in SBIRT was well received and enabled physicians to understand a new potential role. This finding was enhanced by the qualitative interview data in which physicians expressed their satisfaction with training; they were willing to adopt SBIRT and could see the role that primary care generally could play in managing substance use in the United Arab Emirates. Training in managing problematic substance use has proven to be key to changing attitudes and ultimately even the willingness of a workforce to manage groups that can be stigmatised and initially viewed negatively (Matheson et al., Reference Matheson, Thiruvothiyur, Robertson and Bond2016). The willingness of primary care physicians to manage problem substance use was evident. Unfortunately, over time, the positive effect of training on attitudes and belief in self efficacy in SBIRT dropped to pre-training levels. Qualitative interview data indicated that the time taken to implement the study after training and the confusion over remuneration were both contributing factors (Pflanz-Sinclair et al., Reference Pflanz-Sinclair, Bond, Lee, El Kashef, Almarzouqi, Batieha and AlGhaferi2017).
Implementation of SBIRT in a new area
The positive implication from this study is that if SBIRT implementation is managed in a co-ordinated manner then primary care physicians are positive towards their role. Implementation research aims to reduce the gap between what is known to be effective (in this case SBIRT) and how it is actually delivered in a particular health care setting (Bero et al., Reference Bero, Grilli, Grimshaw, Harvey, Oxman and Thomson1998). This SBIRT project can generate useful, generalisable knowledge which will help to narrow this implementation gap and improve effective delivery of a new screening service in primary care in the United Arab Emirates, and more widely in the Middle East.
In conclusion, this first study of its kind in the Middle East, demonstrated that it is possible to implement primary care screening for substance use from the primary care perspective. However, poor patient attendance at follow-up requires investigation.
Acknowledgements
The authors would like to acknowledge all the Primary Care Physicians and patients who participated in this project.
Conflicts of Interest
There are no conflicts of interest for authors C.M., C.B., C.P. and A.J. Authors A.B., A.A., A.E. and H.A. were employed at the NRC who also funded the study. However, these authors did not input into the analysis or interpretation of results.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guidelines on human experimentation (National Rehabilitation Centre, United Arab Emirates and University of Aberdeen, Division of Applied Health Sciences) and with the Helsinki Declaration of 1975, as revised in 2008.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1463423617000640










