Introduction
The World Health Organization (WHO) includes fascioliasis in the list of the NTDs (Neglected Tropical Diseases), among the group of food-borne trematodiases (WHO, 2013). This disease is a parasitic zoonosis caused by two liver fluke species: Fasciola hepatica distributed throughout Europe, Africa, Asia, Oceania and the Americas, and F. gigantica restricted to parts of Africa and Asia (Mas-Coma et al., Reference Mas-Coma, Valero and Bargues2009a). Widely distributed infecting livestock, human infection by Fasciola was considered of only secondary importance until 1990 (Chen and Mott, Reference Chen and Mott1990). Human affection by these trematodes began to show its importance from the following decade, with the progressive description of many human endemic areas and an increase of human infection reports (Mas-Coma et al., Reference Mas-Coma, Valero and Bargues2009a, Reference Mas-Coma, Bargues and Valero2014a).
The impact of this disease is due to its high pathogenicity (Mas-Coma et al., Reference Mas-Coma, Agramunt and Valero2014b) and immune suppression not only during the migratory, invasive or acute phase (Dalton et al., Reference Dalton, Robinson, Mulcahy, O'Neill and Donnelly2013), as previously believed (see review in Chen and Mott, Reference Chen and Mott1990), but also during the long biliary or chronic phase (Girones et al., Reference Girones, Valero, Garcia-Bodelon, Chico-Calero, Punzon, Fresno and Mas-Coma2007) in which almost all of the inhabitants of human endemic areas are diagnosed (Mas-Coma et al., Reference Mas-Coma, Bargues and Valero2014a), as well as in the reinfections in human hyperendemic areas (Valero et al., Reference Valero, Perez-Crespo, Chillon-Marinas, Khoubbane, Quesada, Reguera-Gomez, Mas-Coma, Fresno and Girones2017). Global estimations of around 17 million people infected worldwide (Mas-Coma et al., Reference Mas-Coma, Valero and Bargues2009a) and extreme pathogenicity situations as neurological and ophthalmological affections giving rise to permanent sequelae and even fatal cases (Mas-Coma et al., Reference Mas-Coma, Agramunt and Valero2014b) speak about the public health importance of this disease.
Throughout its worldwide distribution, human fascioliasis shows a very high heterogeneity concerning both disease transmission patterns and epidemiological scenarios linked to a complexity of inter-related aspects which is in need for a multidisciplinarity approach (Mas-Coma et al., Reference Mas-Coma, Valero and Bargues2009a, Reference Mas-Coma, Valero and Bargues2009b). Moreover, the absence of parallelism between human and animal fascioliasis regarding prevalences and intensities should be emphasized. This fact is the consequence of differences in behaviour, habits, traditions and diet according to the different regions, social characteristics and cultures in the world. Among the aspects underlying the aforementioned heterogeneity, the following may be highlighted: (i) parasite adaptation capacities, (ii) fasciolid transmission characteristics, (iii) lymnaeid vector ecological strategies and spreading, (iv) livestock and domestic animal species involved and their management, (v) climate changes, (vi) global changes, (vii) general human behavioural characteristics and (ix) human infection sources.
Fasciolids have proved to be very susceptible to the environmental characteristics (Fuentes et al., Reference Fuentes, Valero, Bargues, Esteban, Angles and Mas-Coma1999, Reference Fuentes, Malone and Mas-Coma2001), which explains why fascioliasis may be influenced by both climate and global changes. Indeed, this trematodiasis presents features which are crucial for a high response capacity to environmental modifications, such as (i) being zoonotic with (ii) low reservoir specificity (Valero and Mas-Coma, Reference Valero and Mas-Coma2000; Valero et al., Reference Valero, Panova, Comes, Fons and Mas-Coma2002) and (iii) freshwater snail vector-borne including (iv) a high number of lymnaeid snail species with transmission capacity (Bargues et al., Reference Bargues, Vigo, Horak, Dvorak, Patzner, Pointier, Jackiewicz, Meier-Brook and Mas-Coma2001). Regarding global change, fascioliasis is influenced by human and animal movements and by anthropogenic disturbances of the external milieu (Mas-Coma et al., Reference Mas-Coma, Valero and Bargues2009b).
Human infection sources are key factors in defining human prevalences and intensities in a given area. It should be considered that metacercariae from different livestock species origins do not show significant differences in definitive host infectivity and moreover metacercariae have a long-term viability allowing old metacercariae of a little more than 1 year, sporadically even up to almost 2 years of age, for a successful infection of the definitive host (Valero and Mas-Coma, Reference Valero and Mas-Coma2000; Valero et al., Reference Valero, Darce, Panova and Mas-Coma2001, Reference Valero, Panova, Comes, Fons and Mas-Coma2002).
Time ago, studies in developed countries, mainly in Europe, suggested that human infection was related to the ingestion of freshwater consumable vegetables carrying attached infective Fasciola metacercariae, such as those usually used in salads and dish accompaniments. Mainly watercress, secondarily dandelion and rarely other metacercariae-carrying aquatic plants for human consumption were noted to be the most important infection sources for humans in Europe and this scenario was accepted to be extrapolable worldwide. From the 1990s, studies on human endemic areas and reports of human infection cases in many different, mostly developing countries (Mas-Coma et al., Reference Mas-Coma, Valero and Bargues2009a, Reference Mas-Coma, Bargues and Valero2014a) have furnished new information indicating that human infection may be the consequence from many different sources not previously considered, and even allowing to discard other human infection sources previously annotated.
The purpose of this article includes an analysis of the human infection risk and related incidence factors, with emphasis on the seasonality, an update of the different human infection sources so far counting with sufficient support, an overview of the methods and techniques to assess these human infection sources, and the individual prevention and general control measures which may be useful to avoid the infection in each one of these sources. It is worth mentioning that these crucial aspects in human fascioliasis have never before been the focus of an extensive appropriate analysis despite their importance in human infection, disease transmission, epidemiology, individual prophylaxis and general control measures. Short and superficial overviews may sometimes be found in textbooks and detailed analyses are very rare and concern only local situations. This is the first time that a complete in-depth analysis of the human infection sources of this disease considering the highly heterogenic worldwide human fascioliasis scenario is made.
Fasciolid transmission
The two species F. hepatica and F. gigantica follow a similar two-host life cycle pattern. It takes about 14–23 weeks and comprises four phases (Mas-Coma and Bargues, Reference Mas-Coma and Bargues1997):
(A) The fluke adult stage infects the large biliary passages and gallbladder of the definitive host, both humans and animals, mainly livestock but also wild herbivores; eggs reach the external milieu by way of bile and intestine; the definitive host is infected by ingestion of metacercariae; metacercariae excyst in the small intestine within 1 h after ingestion, penetrate the host's intestine wall, and appear in the abdominal cavity by about 2 h after ingestion; most reach the liver within 6 days after excystment; in the liver they migrate for 5–6 weeks, preferentially feeding directly on liver tissue; they eventually penetrate into the bile ducts where they become sexually mature; the prepatent period (from the ingestion of metacercariae to the first appearance of the first eggs in the feces) varies according to the host and also depends on the number of the adult flukes in the liver (Valero et al., Reference Valero, De Renzi, Panova, García-Bodelon, Periago, Ordoñez and Mas-Coma2006b); in man, a period of at least 3–4 months is necessary for the flukes to attain sexual maturity; this period is 1–2 weeks longer in F. gigantica (Valero et al., Reference Valero, Bargues, Khoubbane, Artigas, Quesada, Berinde, Ubeira, Mezo, Hernandez, Agramunt and Mas-Coma2016).
(B) The transit between the definitive mammal host and intermediate snail host includes the long resistance phase of the egg and the short active phase of miracidium.
(C) At the intermediate host level, the development includes miracidium penetration into the snail, development of sporocyst and redial generations, production of cercariae and shedding of the latter into water.
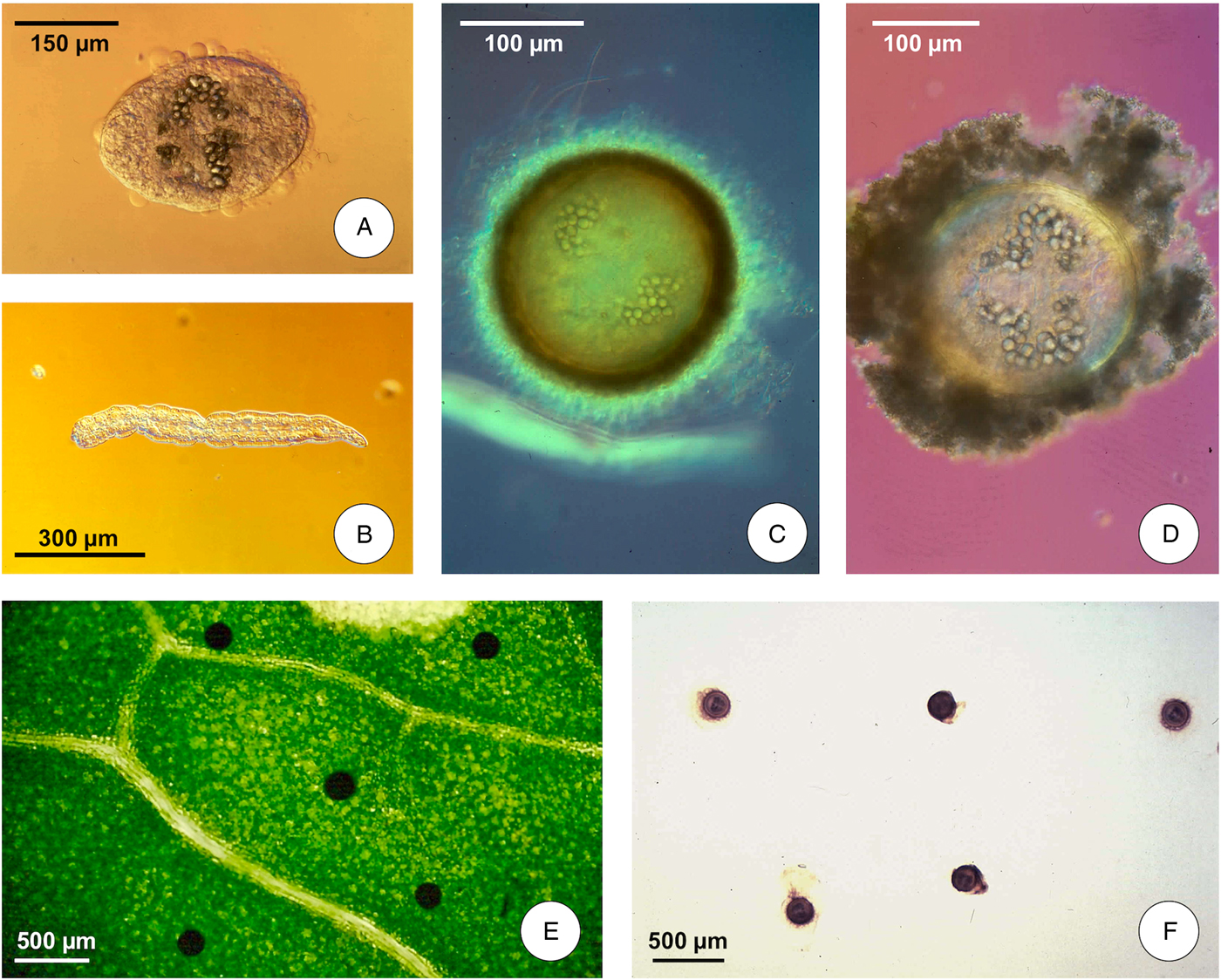
(D) The transit between intermediate snail host and definitive mammal host includes the short swimming phase of cercaria and the long resistance phase of metacercaria until its ingestion by the definitive host; the shedding process takes place independently of light or darkness, between 9 and 26 °C in F. hepatica (at a somewhat higher temperature range in F. gigantica, whose minimum temperature threshold is 16 °C – Afshan et al., Reference Afshan, Fortes-Lima, Artigas, Valero, Qayyum and Mas-Coma2014); cercariae swim for a short time (1 h) until contacting a solid support, mostly leaves of water plants above or below the water line; they then lose their tails and quickly encyst (Fig. 1A, B), changing into round metacercariae (Fig. 1C) attached to the vegetation (Fig. 1E); floating infective metacercarial cysts (Fig. 1D) are also originated at the level of the water surface line (Fig. 1F) (Vareille-Morel et al., Reference Vareille-Morel, Dreyfuss and Rondelaud1993); metacercarial cysts become infective within 24–72 h.

Fig. 1. Life cycle stages of Fasciola hepatica involved in the infection of humans: (A) cercarial body beginning the encystment process; (B) cercarial tail after detachment from cercarial body; (C) metacercarial attached cyst; (D) metacercarial floating cyst; (E) metacercariae attached to a green plant leaf; (F) metacercariae floating in water. (Photographs S. Mas-Coma).
The cercarial shedding process seems to follow an infradaily shedding pattern of 7 days in the daily production during the whole emergence and a circadial rhythm with maximum production between midnight and 1.00 h a.m., as seen in the lymnaeid vector Galba truncatula infected by F. hepatica (Audousset et al., Reference Audousset, Rondelaud, Dreyfuss and Vareille-Morel1989). Higher cercarial productions following different shedding chronobiologies have been seen in the same lymnaeid at very high altitude and by other Galba/Fossaria species in the lowlands (Bargues et al., Reference Bargues, Gayo, Sanchis, Artigas, Khoubbane, Birriel and Mas-Coma2017).
Human infection risk
Epidemiological scenarios and transmission patterns
The risk of human infection depends on the fascioliasis transmission rate in the area in question and on its intra- and interannual rate variability linked to climatic factor variations. The marked heterogeneity of human fascioliasis regarding different epidemiological scenarios and transmission patterns throughout the world should be noted in this regard. It may be concluded that well-known situations and patterns of fascioliasis may not always explain the disease characteristics in a given area.
The transmission rate may be inferred from local prevalences and intensities in the inhabitants but also from livestock living in the area. Indeed, different epidemiological situations have been distinguished in human fascioliasis. The classification of epidemiological scenarios proposed by Mas-Coma et al. (Reference Mas-Coma, Esteban and Bargues1999a, Reference Mas-Coma, Valero and Bargues2009a) still appears to be fully valid and useful. This classification includes: (i) imported cases; (ii) authochthonous, isolated, non-constant cases; (iii) three different human endemic situations, comprising hypoendemic [prevalence of <1%; arithmetic mean intensity <50 eggs per gram (epg) of feces], mesoendemic (prevalence of between 1 and 10%; 5–15-year-old children may present higher prevalences; arithmetic mean intensity in human communities may reach 50 and 300 epg), and hyperendemic (prevalence of more than 10%; 5–15-year-old children usually present higher prevalences; arithmetic mean intensity in human communities may reach values higher than 300 epg); and (iv) two different human epidemic situations (epidemics in animal but non-human endemic areas, and epidemics in human endemic areas).
The intra- and interannual variability of the transmission rate is due to the marked dependence of fasciolid-free larval stages (aforementioned phases B and D) and intramolluscan larval stages (phase C) on the environmental characteristics (Ollerenshaw and Smith, Reference Ollerenshaw and Smith1969; Ross, Reference Ross1970; Ollerenshaw, Reference Ollerenshaw1971; Fuentes et al., Reference Fuentes, Valero, Bargues, Esteban, Angles and Mas-Coma1999), namely: surface water availability whether from rainfall or from any freshwater body (rivers, streams, lagoons, lakes, subsoil efflorescences, irrigation canals, fountains, etc.) to allow for the presence, development and population dynamics of the lymnaeid vectors (Mas-Coma et al., Reference Mas-Coma, Angles, Esteban, Bargues, Buchon, Franken and Strauss1999b) and mainly air and water temperature as main factor. Minimum and maximum larval development temperature thresholds are different for F. hepatica and F. gigantica (Afshan et al., Reference Afshan, Fortes-Lima, Artigas, Valero, Qayyum and Mas-Coma2014).
Different transmission patterns have been distinguished within the various human endemic areas (Mas-Coma, Reference Mas-Coma2005): (a) a very high altitude pattern related to only F. hepatica in Andean countries, which includes two subpatterns (the altiplanic pattern with transmission throughout the whole year, and the valley pattern, with seasonality and prevalences and intensities related to altitude); (b) a Caribbean insular pattern, with reduced but repeated outbreaks in human hypoendemic areas; (c) a pattern related to Afro-Mediterranean lowlands, including overlapping F. hepatica and F. gigantica, and where seasonality is typical; (d) a pattern occurring in areas surrounding the Caspian, including human hypoendemic areas in which large epidemics occur, occasionally involving up to 10 000 people and with overlapping of F. hepatica and F. gigantica; (e) a pattern recently detected in Vietnam, related to only/mainly F. gigantica, linked to lowland areas and able to give rise to large human epidemics (De et al., Reference De, Murrell, Cong, Cam, Chau, Toan and Dalsgaard2003; The and Nawa, Reference The and Nawa2005; Le et al., Reference Le, De, Agatsuma, Nguyen, Nguyen, McManus and Blair2008); and (f) a new pattern very recently found in Argentina corresponding to isolated foci in desertic-arid and semi-arid conditions where transmission factors are concentrated and seasonal transmission depends on the timely overlap of appropriate temperature and river water availability (Bargues et al., Reference Bargues, Malandrini, Artigas, Soria, Velasquez, Carnevale, Mateo, Khoubbane and Mas-Coma2016).
Seasonality
Seasonality is an important infection risk factor to take into account. Seasonal incidence is pronouncedly determined by climate factors, mainly temperature and rainfall. Human infection has been more frequently observed in the years with heavy rainfall (Ripert et al., Reference Ripert, Tribouley, Luong Dinh Giap, Combes, Laborde and Bourianne1988), and in, for instance, Western Europe, fascioliasis is referred to as markedly seasonal, with a high percentage (80.9%) of the cases showing the onset of the disease in the autumn months (Garcia-Rodriguez et al., Reference Garcia-Rodriguez, Martin Sanchez, Fernandez Gorostarzu and Garcia Luis1985), although the relatively long survival of metacercariae (Valero and Mas-Coma, Reference Valero and Mas-Coma2000) explain sporadic individual infections throughout other seasons of the year. Sometimes the seasonality is related to the transmitting plants, most human cases occurring during the watercress season. In Iran, past epidemics of human fascioliasis have been linked to the Ramadan; this period typically driving people to increase the consumption of vegetables.
Almost all fascioliasis endemic areas follow a seasonal transmission of the disease, which is nothing else than the translation of the lymnaeid vector population dynamics in the area in question and which in its turn depends on the local climatic characteristics. Lymnaeids have greatly differing ecological and ethological characteristics depending on the species. Factors such as the type of water collection habitats, lymnaeid population dynamics, different temperature thresholds of the different lymnaeid vector species and their local geographical strains, seasonality or susceptibility regarding liver fluke infection are crucially important for fascioliasis. All this indicates that similarly as known in other vector-borne parasitic diseases, lymnaeids may constitute excellent markers of the disease, useful for differentiating between the various human fascioliasis scenarios and patterns, and consequently also as determinants for the design of appropriate control strategies.
From a global perspective, three main types of transmission seasonality may be distinguished according primarily to latitude and secondarily altitude:
(A) Permanent, year-long transmission: this occurs in zones where mean monthly temperatures fluctuate scarcely and are kept within the minimum and maximim fasciolid larval stage development thresholds throughout the year; such a transmission appears in foci where lymnaeid vectors are adapted to permanent water bodies as observed in southern Europe (Valero et al., Reference Valero, Marti, Marcos, Robles and Mas-Coma1998) and Mediterranean islands (Oviedo et al., Reference Oviedo, Mas-Coma, Dominici and Roche1992); a similar situation appears in Cambodia (Tum et al., Reference Tum, Puotinen and Copeman2004, Reference Tum, Puotinen, Skerratt, Chan and Sothoeun2007); an effect of the very high altitude of around 4000 m a.s.l. is observed in Andean areas as the Northern Bolivian Altiplano where the high evapotranspiration leads lymnaeids to only inhabit permanent water bodies and daily temperatures counteract the negative effects of the low night temperatures (Fuentes et al., Reference Fuentes, Valero, Bargues, Esteban, Angles and Mas-Coma1999; Mas-Coma et al., Reference Mas-Coma, Angles, Esteban, Bargues, Buchon, Franken and Strauss1999b); a higher number of redial generations (up to four) is typical in such places (Mas-Coma et al., Reference Mas-Coma, Funatsu and Bargues2001).
Where seasonality occurs, temporary transmission is mainly related to lymnaeid vectors able to quickly multiply and colonize temporary water bodies from rainfall and to aestivate and hibernate during the non-appropriate periods.
(B) Monoseasonal transmission: this occurs in extreme latitudes, where only an appropriate temperature month window appears throughout the year, whether in usually northern or southern too cold areas as the Patagonia and mountainous areas, or in more tropical, too warm areas. This may be, for instance, the situation of warm areas in south-central Asia where the monsoon period concentrate the rainfall and the absence of irrigation systems does not allow for another yearly transmission period (Afshan et al., Reference Afshan, Fortes-Lima, Artigas, Valero, Qayyum and Mas-Coma2014).
(C) Biseasonal transmission: this is the typical situation in Europe, given areas of the USA and also Australia. This biseasonal model includes low transmission in spring and very high transmission in autumn, with highest animal incidences from August–September expanding even up to December–February (Ross, Reference Ross1967, Reference Ross1977; Urquhart et al., Reference Urquhart, Doyle and Jennings1970; Over and Dijkstra, Reference Over and Dijkstra1975; Meek and Morris, Reference Meek and Morris1979; Shaka and Nansen, Reference Shaka and Nansen1979; Craig Hoover et al., Reference Craig Hoover, Lincoln, Hall and Wescoo1984; Mage, Reference Mage1989a, Reference Mage1989b). This biseasonality inverses in other areas where the highest incidence appears in the first half of the year (Harris and Charleston, Reference Harris and Charleston1976; Craig and Bell, Reference Craig and Bell1978; Malone et al., Reference Malone, Loyacano, Hugh-Jones and Corkum1984/85; Boyce and Courtney, Reference Boyce and Courtney1990).
Interestingly, however, a wide global analysis of existing data shows that there is no marked seasonal incidence, human infections occurring nearly throughout the year (Chen and Mott, Reference Chen and Mott1990). This means that metacercarial survival, viability and infectivity during several months if kept under appropriate conditions, mainly sufficient humidity, may qualitatively mask the human infection seasonal distribution due to the higher infectivity of the recent, young metacercariae.
Fascioliasis has been many times reported to be linked to man-made irrigation areas. Everywhere, but more usually in rural areas of developing countries, livestock is freely grazing in irrigated plant cultures and also in their neighbouring irrigation canals where lymnaeids are frequently found. The frequent presence of lymnaeids in rice fields in Northern Africa, Asia and Southern Europe is a good example (Valero et al., Reference Valero, Marti, Marcos, Robles and Mas-Coma1998). Thus, the different local traditions in the timely artificial floodings of the rice field managements from the irrigation systems modify the fascioliasis transmission seasonality.
In the Punjab, Pakistan, it has been recently proved that human and animal fascioliasis transmission is biseasonal (Qureshi et al., Reference Qureshi, Tanveer and Mas-Coma2016), with one summer peak related to monsoons rainfall and another winter peak related to artificial irrigation (Afshan et al., Reference Afshan, Fortes-Lima, Artigas, Valero, Qayyum and Mas-Coma2014). In that endemic area, a great amount of water supplied by the Indus basin river system is used for irrigation by means of a very large irrigation system including dams, barrages and a canal network of 60 000 km constructed during British colonial years (Afshan et al., Reference Afshan, Fortes-Lima, Artigas, Valero, Qayyum and Mas-Coma2014). Such an immense irrigation system is used in great part for the cultivation of crops.
Community, familial and social factors in infection risk
Fascioliasis is predominantly a rural disease because human infection risk is in the field where the disease transmission occurs in freshwater bodies inhabited by the lymnaeid vectors. A thorough epidemiological study in the highest human hyperendemic area known, the Northern Bolivian Altiplano, proved that prevalence and intensity of infection in the communities show a direct correlation with, and are therefore dependent on, the distance of the village from the closest water collection inhabited by lymnaeid snail vectors (Mas-Coma et al., Reference Mas-Coma, Angles, Esteban, Bargues, Buchon, Franken and Strauss1999b). In a developed country as France, human infection in 10 000 reports, happened during the 1956–1982 period, correlated well with the zones for cattle and sheep husbandry (Gaillet et al., Reference Gaillet, Liance, Rivollet and Houin1983).
Human infection in urban settlements occurs only sporadically due to consumption, mainly at home but also very rarely at restaurants and hotels, of metacercariae-carrying vegetables acquired in an uncontrolled market to where they were transported from the field. This does however not exclude the possibility of urban inhabitants to become infected in field trips.
The infection distribution by sex appears to be very similar in several areas, as in Europe, although in human hyperendemic areas the females show higher infection rates, whether prevalences as in Egypt (Farag et al., Reference Farag, Barakat, Ragab and Omar1979; Esteban et al., Reference Esteban, Gonzalez, Curtale, Muñoz-Antoli, Valero, Bargues, El Sayed, El Wakeel, Abdel-Wahab, Montresor, Engels, Savioli and Mas-Coma2003), or intensities as in Bolivia (Esteban et al., Reference Esteban, Flores, Aguirre, Strauss, Angles and Mas-Coma1997a, Reference Esteban, Flores, Angles, Strauss, Aguirre and Mas-Coma1997b, Reference Esteban, Flores, Angles and Mas-Coma1999). Regarding age relationships, all age groups can be affected, although in human hyperendemic areas children appear to be the most infected (Esteban et al., Reference Esteban, Flores, Angles and Mas-Coma1999, Reference Esteban, Gonzalez, Curtale, Muñoz-Antoli, Valero, Bargues, El Sayed, El Wakeel, Abdel-Wahab, Montresor, Engels, Savioli and Mas-Coma2003; Gonzalez et al., Reference Gonzalez, Esteban, Bargues, Valero, Ortiz, Naquira and Mas-Coma2011; Zumaquero-Rios et al., Reference Zumaquero-Rios, Sarracent-Perez, Rojas-Garcia, Rojas-Rivero, Martinez-Tovilla, Valero and Mas-Coma2013).
The incidence of infection is significantly aggregated within family groups because the family shares the same contaminated food and/or water, as it has been observed in for instance Spain (Gallardo et al., Reference Gallardo, Saenz and Enriquez1976; Garcia-Rodriguez et al., Reference Garcia-Rodriguez, Martin Sanchez, Fernandez Gorostarzu and Garcia Luis1985; Rodríguez Hernández et al., Reference Rodríguez Hernández, Canut Blasco, Brezmes Valdivieso, Martín Arribas, Arias Paciencia, Santana Rodríguez and Martín Sánchez1998), Germany (Bechtel et al., Reference Bechtel, Feucht, Held and Nothdurft1992), Egypt (Farag et al., Reference Farag, Barakat, Ragab and Omar1979) and Peru (Marcos et al., Reference Marcos, Maco, Terashima, Samalvides, Espinoza and Gotuzzo2005). Familial clustering has been also found in patients from the French island of Corsica (Gil-Benito et al., Reference Gil-Benito, Mas-Coma, Quilici and Ciolkovitch1991a) and in community-based surveys in the Northern Bolivian Altiplano (Mas-Coma et al., unpublished data). In a community-based survey in Egypt, among 25 families with at least one infected person, 20% had two members infected and another 20% had three members infected (Farag et al., Reference Farag, Barakat, Ragab and Omar1979).
Human infection sources
Studies performed in many countries in the last three decades have demonstrated that there are more sources of human infection than the very few distinguished time ago and traditionally evoked in textbooks (Mas-Coma, Reference Mas-Coma, Cotruvo, Dufour, Rees, Bartram, Carr, Cliver, Craun, Fayer and Gannon2004). Interestingly, evidence indicates that fascioliasis belongs to the rare diseases which may infect humans by both food and drinking sources. Hence, dietary and drinking habits of the human populations are very important in fascioliasis.
Ingestion of freshwater wild plants
Plant markers of transmission foci
Both field studies and experimental work in the laboratory indicate that fasciolid cercariae do not show preferences for one or other type of aquatic vegetables, plants selected by them to attach and encyst becoming metacercariae depending on the ecology of the lymnaeid vectors in each endemic area. Given that lymnaeids show evident preferences for stagnant or only very slowly running waters, and considering the limited swimming capacity of cercariae, the vegetables selected are those growing in the water body where the lymnaeids are present. There are only two factors which have a clear impact by determining the absence of the lymnaeid vectors, namely the existence of salt and shadow. Lymnaeid vectors do not inhabit brackish waters because they do not tolerate even low salt concentrations, nor water collections (or parts of them) which are under permanent shadow impeding sunshine to allow for the growth of the freshwater algae from which lymnaeids like to feet (Mas-Coma et al., Reference Mas-Coma, Angles, Esteban, Bargues, Buchon, Franken and Strauss1999b).
In Europe, although the main lymnaeid vector G. truncatula does not show any direct relationship with the local plant species combination in the water collection, the presence of given plant groups appears to be a good sign of the existence of this lymnaeid (Over, Reference Over1962).
Plant combinations having seen as potential markers are Glyceria fluitans and Glyceria plicata (Floating Sweet-grass or water managrass, perennial grass species occurring in wet areas such as ditches, riverbanks and ponds), Alopecurus geniculastus (commonly known as water foxtail or marsh foxtail, a grass species which grows in moist areas) and Ranunculus repens (creeping buttercup or creeping crowfoot, an herbaceous perennial plant very common in damp places, ditches and flooded areas). In other localities, the combination includes Veronica beccabunga (European speedwell, a succulent herb which grows on the margins of brooks and ditches), Glyceria declinata (waxy mannagrass or low glyceria, a small sweet-grass which invades deep vernal pools, swales, ditches and stock ponds), Juncus inflexus (a rush) and the aforementioned R. repens (Over, Reference Over1962). Galba truncatula does not inhabit brackisch waters such as those of the Lake Titicaca (Mas-Coma et al., Reference Mas-Coma, Angles, Esteban, Bargues, Buchon, Franken and Strauss1999b) and the Caspian Sea (Ashrafi et al., Reference Ashrafi, Valero, Peixoto, Artigas, Panova and Mas-Coma2015), but may be present in somehow salty environments presenting Juncus gerardii (blackgrass, black needle rush or saltmarsh rush occurring along the shorelines of areas once flooded by the sea), Glaux maritima (Black saltwort growing in humid habitat or water along seashore environments), Carex otrubae (the false fox-sedge) and Festuca arundinacea (a grass commonly known as tall fescue found in damp grasslands, river banks and in coastal seashore locations) (Over, Reference Over1962). In grasslands where sheep use to feet, the presence of Ranunculus flammula (lesser spearwort, greater creeping spearwort or banewort, a poisonous species of perennial herbaceous plant) appears to be a good indicator (Over, Reference Over1962).
Many other authors have furnished lists of plants which may be used as indicators of the habitats of G. truncatula, such as in Germany (Patzer, Reference Patzer1927; Mehl, Reference Mehl1932) and the UK (de Vries, Reference de Vries1945; Roberts, Reference Roberts1950). In France, rush species as Juncus acutiflorus and J. effusus have already been emphasized (Ghestem et al., Reference Ghestem, Morel-Vareille, Rondelaud and Vilks1974; Gaultier et al., Reference Gaultier, Rondelaud, Botineau and Ghestem1994; Guy et al., Reference Guy, Rondelaud, Botineau, Dreyfuss and Ghestem1996), with J. acutiflorus as the best marker in grasslands and G. fluitans in the banks of streams and ponds (Dreyfuss et al., Reference Dreyfuss, Vareille-Morel and Rondelaud1997; Hourdin et al., Reference Hourdin, Vignoles, Dreyfuss and Rondelaud2006; Rondelaud et al., Reference Rondelaud, Hourdin, Vignoles, Dreyfuss and Cabaret2011). However, in the Northern Bolivian Altiplano hyperendemic area, where the disease transmission is assured by only G. truncatula of European origin, a thorough field study could not establish any positive relationship between freshwater plant combinations and the presence of this lymnaeid vector, probably due to the extreme conditions of the very high altitude (3800–4100 m a.s.l.) of this area (Mas-Coma et al., Reference Mas-Coma, Angles, Esteban, Bargues, Buchon, Franken and Strauss1999b).
The lack of direct relationship of the presence of the lymnaeid vector and the vegetation inhabiting the water collection does not mean, however, that outer characteristics of the parts of the plant in contact with the water may offer more or less facilities for cercarial attachment. Thus, an experimental study in Egypt indicated that cercariae of Fasciola spp. prefer to encyst on dark green leaves with the hairy epidermis, followed by leaves with a serrated and mamillated epidermis, whereas plants with a smooth chitinized epidermal surface are those to which the fewest metacercariae attach (WHO, 1995).
Summing up, among the freshwater wild plants carrying metacercariae, their more or less important role in the transmission to humans will mainly depend on the diet and traditions of the humans inhabiting the area in question. Freshwater wild plants are an important human infection source in animal endemic areas and also in given human endemic areas. Among the vegetables incriminated in human infection, freshwater plant species differ according to geographical zones and human dietary habits. Moreover, plant species involved are not necessarily the same in subjects infected ‘at table’ (through vegetables making part of the normal diet) than in subjects ‘infected in the field’ (ingestion, sucking, chewing or stripping with the teeth of vegetables directly taken from the nature and which may not necessarily make part of the usual human diet).
Watercress
Anamnesis in most reports of human infection uses to refer to watercress as the most probable source of the infection of the patient. However, the general term watercress includes different aquatic species such as Nasturtium officinale (common watercress), N. or Roripa silvestris and Roripa amphibia (wild watercress). Wild watercress has been reported as the main source of human infection in areas where fascioliasis in domestic animals is highly endemic.
Watercress is a green leafy vegetable that grows in most temperate and tropical areas of the world. It is the vegetable most involved in patients diagnosed in countries, such as in the USA (Price et al., Reference Price, Tuazon and Simon1993). In Latin America, wild watercress has been involved in patient infection in many countries, as Mexico (Zumaquero-Rios et al., Reference Zumaquero-Rios, Sarracent-Perez, Rojas-Garcia, Rojas-Rivero, Martinez-Tovilla, Valero and Mas-Coma2013), Cuba (Diaz et al., Reference Diaz, Pina, Lastre, Rivera and Perez1990; Gonzalez et al., Reference Gonzalez, Perez, Perez, Gonzalez de la Torre, Lastre, Brito and Diaz1987; Brito et al., Reference Brito, Olazabal, Pérez, Lastre, González and Pérez1987), Dominican Republic (Noyer et al., Reference Noyer, Coyle, Werner, Dupouy-Camet, Tanowitz and Wittner2002), Venezuela (Rodriguez and Gonzalez, Reference Rodriguez and Gonzalez1975; Abdul-Hadi et al., Reference Abdul-Hadi, Contreras, Tombazzi, Alvarez and Melendez1996), Peru (Blancas et al., Reference Blancas, Terashima, Maguiña, Lujan, Alvarez and Casanova2004) and Argentina (Mera y Sierra et al., Reference Mera y Sierra, Agramunt, Cuervo and Mas-Coma2011).
In Argentina, several outbreaks appear related to the most common risk factor of ingestion of watercress naturally growing along the river- and stream-beds picked during recreational, weekend or holiday activities. Many of these field excursions are undertaken by a family or as a group activity. A very large number of villages and towns play an important role in these recreational activities. These recreational areas attended by thousands of tourists, campers or weekend visitors present water collections inhabited by lymnaeid vectors and where animals show infection by F. hepatica (Mera y Sierra et al., Reference Mera y Sierra, Agramunt, Cuervo and Mas-Coma2011).
Regarding Europe, watercress consumption appeared linked to liver fluke infection in 69.3% of the fascioliasis patients in Spain (Garcia-Rodriguez et al., Reference Garcia-Rodriguez, Martin Sanchez, Fernandez Gorostarzu and Garcia Luis1985) or even in almost all patients (Arjona et al., Reference Arjona, Riancho, Aguado, Salesa and Gonzalez-Macias1995). In France, wild watercress proved to be the main infection source not only in given areas (Rondelaud, Reference Rondelaud1978, Reference Rondelaud1980) but also in the analysis of 10 000 human cases reported between 1956 and 1982 from around the country (Gaillet et al., Reference Gaillet, Liance, Rivollet and Houin1983). This wild freshwater vegetable is repeatedly noted as the infection source in epidemics, whether relatively wide epidemics or smaller familial outbreaks (Fig. 3C, D) already a long time ago (Bouysset et al., Reference Bouysset, Joinaux and Philippe1943) and even in cases of children (Giraud et al., Reference Giraud, Orsini, Mangiapan and Louchet1955). Watercress appears similarly underlying human infection in other European countries as UK (Hardman et al., Reference Hardman, Jones and Davies1970) and Irland (LaPook et al., Reference LaPook, Magun, Nickerson and Meltzer2000).
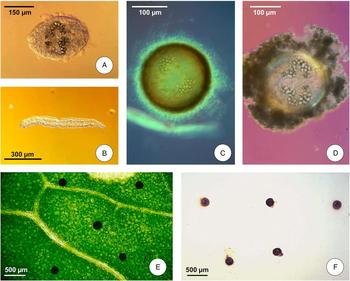
In Asia, watercress appears involved in human infection throughout, from Turkey (Gulsen et al., Reference Gulsen, Savas, Koruk, Kadayifci and Demirci2006) in the West up to Thailand in the South East (Wong et al., Reference Wong, Peura, Mutter, Heit, Birns and Johnson1985). In Iran, wild watercress is inhabited by G. truncatula in the streams of the Iranian mountains (Fig. 2A, B, C). Similarly occurs in Australia (Wood et al., Reference Wood, Stephens and Porter1975; Croese et al., Reference Croese, Chapman and Gallagher1982). In Africa, human infections with F. gigantica are believed to be caused by ingestion of watercress in Rwanda and Burundi (Janssens et al., Reference Janssens, Fain, Limbros, De Muynck, Biemans, Van Meirvenne and De Mulder1968).

Fig. 2. Freshwater plants involved in Fasciola transmission: (A) the one-row yellowcress Nasturtium microphyllum in the Zagros mountains close to Yasuj city, Iran; (B) Galba truncatula is frequently associated to this cress; (C) wild vegetables linked to G. truncatula snails in the Talesh mountains of Guilan, Iran; (D) wild watercress in the Northern Bolivian Altiplano; (E) the small rush Juncus ebracteatus is usually sucked and chewed by Altiplano children; (F) edible algae and Nostoc cyanobacteriae are consumed by the Altiplano Aymara inhabitants; (G) the totora Schoenoplectus californicus ssp. tatora inhabiting bank waters of Lake Titicaca, is not associated to lymnaeids due to its noxious secretions; (H) other freshwater plants appear colonized by lymnaeids besides rapid running stream waters in Dominican Republic mountains. (Photographs S. Mas-Coma).
Other freshwater wild plants
Other such plants have been seen to be involved in the transmission of the disease to humans. Aquatic vegetables other than watercress which have been reported as vehicles of human infection are mainly Taraxacum dens leonis or Taraxacum gr. officinale (dandelion leaves) as in France (Garin et al., Reference Garin, Ronin and Ziegler1944; Rondelaud, Reference Rondelaud1980) and Argentina (Mera y Sierra et al., Reference Mera y Sierra, Agramunt, Cuervo and Mas-Coma2011), Valerianella olitoria (lamb's lettuce) in central France (Rondelaud, Reference Rondelaud1980) and Mentha viridis (spearmint) (Fig. 3A) (Mas-Coma and Bargues, Reference Mas-Coma and Bargues1997; Mas-Coma et al., Reference Mas-Coma, Angles, Esteban, Bargues, Buchon, Franken and Strauss1999b). Wild common sorrel, Rumex acetosa, also known as spinach dock, collected in a swampy meadow appeared to be the source of infection of two sister girls in France (Mohr et al., Reference Mohr, Berka, Knüttgen and Ohr1951). The latter is a slender herbaceous perennial plant that has juicy stems and edible, arrow-shaped leaves and is common in grassland habitats (also cultivated as a garden herb and consumed raw as a salad vegetable).

Fig. 3. Freshwater plant cultures: (A) spearmint Mentha spp. use to live on loamy soils flooded by water where it is colonized by lymnaeid snails, as in Valencia, Spain; (B) plants needing intensive irrigation are consumed in the Nile Delta region; (C) unusual mountainous area in Corsica island, France, where a family outbreak occurred; (D) origin of this outbreak in a garden watercress irrigation canalized from a neighbouring lymnaeid-contaminated spring water; (E) large cultures of water morning glory or water spinach besides Quy Nhon, Vietnam; (F) pond with water caltrop Trapa bispinosa inhabited by lymnaeid snail vectors close to urban setting in southern Taiwan; (G) typical south Asian pond besides dwelling presenting floating water lily (Nymphaea sp.); (H) lymnaeid snail vectors attached to a water lily leaf. (Photographs S. Mas-Coma).
In Iran, several species of wild grown aquatic and/or semi-aquatic plants are a main part of the common human diet in many areas, especially in the endemic region of Guilan Province, the most important zone of human fascioliasis in the country. The species Mentha pulegium, Mentha piperita and Eryngium caucasicum are the main species that have been implicated in human fascioliasis transmission in the Guilan endemic province (Asmar et al., Reference Asmar, Milaninia, Amir-Khani, Yadegari, Forghan-Parast, Nahravanian, Piazak, Esmaeili, Hovanesian and Valadkhani1991; Forghan-Parast et al., Reference Forghan-Parast, Yadegari and Asmar1993; Massoud, Reference Massoud1998). In other Iranian provinces where human fascioliasis has been reported, several species of aquatic plants have been noted to be involved in the disease transmission. Thus, in the southwestern Yasuj district and rural areas of Boyer-Ahmad township the vegetable noted to be involved was the one-row yellowcress Nasturtium microphyllum (locally named ‘bakaloo’ or ‘boolaghuti’) (Fig. 2A, B), Mentha longifolia (known as ‘pooneh’) and spearmint (Sarkari et al., Reference Sarkari, Ghobakhloo, Moshfea and Eilami2012; Hosseini et al., Reference Hosseini, Sarkari, Moshfe, Motazedian and Abdolahi Khabisi2015). In the Mazandaran Province at the seashore of the Caspian Sea, reference has been made to Eryngium spp. and Mentha spp. (Moghaddam et al., Reference Moghaddam, Massoud, Mahmoodi, Mahvi, Periago, Artigas, Fuentes, Bargues and Mas-Coma2004). In the western Kermanshah Province, Nasturtium spp. and Falcaria vulgaris (locally known as ‘paghaze’) have been mentioned (Emami Al-Agha and Athari, Reference Emami Al-Agha and Athari1995). Interestingly, moreover, a 44% of raw vegetables, including spearmint, were found to be contaminated by eggs of Fasciola sp. in Iran (Abdi et al., Reference Abdi, Farhadi, Aghace and Sayehmiri2014).
In the Bolivian Altiplano human hyperendemic area, different freshwater plants have been found carrying metacercariae: 56.3% Compositae; 50.9% Eleocharis sp.; 12.0% Senicio sp.; 10.3% Vallisneria sp.; 3.3% Scirpus sp.; 2.6% Ranunculaceae. In this Andean hyperendemic area, the reports suggest that human infection is related to traditional consumption of uncooked aquatic plants, including (care should be taken with Aymara terms because of usual confusion by Altiplano inhabitants): watercress, berros or ‘okororo’ (Mimulus glabratus and Nasturtium officinale – Scrophulariaceae) (Fig. 2D); matara (Juncus andicola – Juncaceae); totorilla or ‘kosko-oskosko’ (Juncus ebracteatus – Juncaceae) (Fig. 2E); edible algae as cochayuyo or ‘llayta’ (Porphyra purpurea – Chlorophyta) and similar vegetables as Nostoc sp. (Cyanobacteria) (Fig. 2F); and many others (Mas-Coma et al., Reference Mas-Coma, Angles, Strauss, Esteban, Oviedo and Buchon1995, Reference Mas-Coma, Angles, Esteban, Bargues, Buchon, Franken and Strauss1999b; Esteban et al., Reference Esteban, Flores, Aguirre, Strauss, Angles and Mas-Coma1997a). Regarding the so-called totora or ‘chullu’ (Schoenoplectus californicus ssp. tatora – Cyperaceae) (Fig. 2G), frequently referred to by the Altiplano inhabitants, a negative association between presence of lymnaeids and presence of this plant in the same water collection was observed, most probably because of the noxious secretions of its roots (Mas-Coma et al., Reference Mas-Coma, Angles, Esteban, Bargues, Buchon, Franken and Strauss1999b). Among the numerous aquatic and semi-aquatic plant species found in water collections presenting lymnaeids in the Altiplano, mainly J. ebracteatus and M. glabratus and secondarily Nostoc sp. are infection sources for human adults. Concerning the transmission to children, it should be considered that in this endemic very high altitude area, children are malnourished and from an early age many of them help their parents in agricultural activities and in the tending of animals. The many hours spent away from home, in turn, leads them to eat, suck or chew many wild vegetables, which may constitute vehicles that enable the access of metacercariae to their alimentary tract (Esteban et al., Reference Esteban, Flores, Aguirre, Strauss, Angles and Mas-Coma1997a). Among the many other wild freshwater plant species involved in the infection of children, Hydrocotyle ranunculoides, Eleocharis spp. Rorippa spp., other Juncaceae and Scrophulariaceae, Compositae, etc., should be counted (Mas-Coma et al., Reference Mas-Coma, Angles, Esteban, Bargues, Buchon, Franken and Strauss1999b).
In Argentina, two patients were mentioned to have chewed blades of grass that grew on a riverbank (Mera y Sierra et al., Reference Mera y Sierra, Agramunt, Cuervo and Mas-Coma2011). This appears to be a problem mainly with children, as they put into mouth all kind of objects and above all wild plants collected in nature during walks, as was the case of a girl frequently sucking wild grass hazardly collected along walks and diagnosed in France (Martin et al., Reference Martin, Le, Sureau, Babouot and Bourcart1944). The high risk of chewing wild grass by children was highlighted in a recent questionnaire survey made in Baños del Inca, Cajamarca, Peru (Rodríguez et al., Reference Rodríguez, Rivera, Del Valle, Cerna, Hoban, Chilón and Ortiz2018). Other freshwater plants appeared colonized by lymnaeid snails besides rapid running stream waters in the mountains of the Dominican Republic (Fig. 2H).
In Hawaii, human infection was noted to take place by the accidental ingestion of raw vegetation, including watercress (Alicata and Bonnet, Reference Alicata and Bonnet1956) containing encysted metacercariae, particularly in areas where infected cattle were permitted to roam (Stemmermann, Reference Stemmermann1953a, Reference Stemmermann1953b).
In Asia, a human fascioliasis case report in Thailand in which the water morning glory or water spinach (Ipomoea aquatica) was suspected to be the origin of the patient's infection (Wong et al., Reference Wong, Peura, Mutter, Heit, Birns and Johnson1985) suggested the appropriateness to enlarge the spectrum of freshwater plants involved in the transmission of Fasciola to humans in Asian countries by including the vegetables always considered to underlie human infection by Fasciolopsis buski, another trematode in whose life cycle cercariae behave similarly to those of Fasciola (Mas-Coma et al., Reference Mas-Coma, Bargues and Valero2005). Indeed, this is supported by the coexistence of both Fasciola in ruminants and Fasciolopsis in pigs in the same area of human infection by Fasciola (Manning and Ratanarat, Reference Manning and Ratanarat1969) and by the usual coexistence of the lymnaeid vectors of Fasciola and the planorbid freshwater snails transmitting F. buski in the same water collections. Therefore, the following plant species may be considered: the water caltrop (Trapa natans in China, T. bispinosa in Taiwan and T. bicornis in Bangladesh and Thailand) (Fig. 3F), the water chestnut (Eliocharis tuberosa), the water lotus (Nymphaea lotus), water bamboo (Zizania aquatica) and other freshwater vegetation including eelgrass or tape grass (Valisneria spp.), floating fern, watermoss or water butterfly wings (Salvinia natans), common duckmeat or greater duckweed (Spirodela polyrhiza = Lemna polyrhiza), water hyacinth (Eichhornia crassipes), water lily (Nymphaea sp.), watercress, gankola (Otelia sp.), and the aformentioned water morning glory or water spinach (I. aquatica) (WHO, 1995; Mas-Coma et al., Reference Mas-Coma, Bargues and Valero2005). In Thailand, the species frequently consumed are water caltrop (Trapa hicornis), water hyacinth (E. crassipes), lotus (N. lotus), water mimosa (Neptunia oleraced) and water spinach (I. aquatica) (WHO, 1995). Most of these edible plants grow near the houses, that is, where pollution takes place (Manning and Ratanarat, Reference Manning and Ratanarat1970). Moreover, ‘night soil’ (human excrement collected from latrines) is used to fertilize fish ponds and to feed fish where these plants are present, in that way enhancing disease transmission (Cross, Reference Cross1984).
In Africa, human infections with F. gigantica may also occur after chewing infested grass or green rice (Janssens et al., Reference Janssens, Fain, Limbros, De Muynck, Biemans, Van Meirvenne and De Mulder1968). It is believed that one of the reasons why human fascioliasis is rare in southern Africa may be the dietary habits of the Africans in this area (Fig. 3B) where water plants do not seem to be an important source of food or relish and, in any case, are mostly eaten cooked (Gelfand, Reference Gelfand1971; Goldsmid, Reference Goldsmid1975). However, in Malawi, it has been pointed out that some vegetable plants are eaten uncooked, including cabbage, tanaposi and mnadzi, and these may serve as sources of infection in swampy areas. It is also suggested that sugar cane grown in swampy areas may serve as a source of metacercarial ingestion, the cane commonly being stripped by Africans with their teeth (Speckhart, Reference Speckhart1969).
Wild plants sold in urban markets
In given reports, information provided by the patient indicated that the infection was from watercress bought in an urban market or bazaar, as seen in Turkey (Kaya et al., Reference Kaya, Demirci, Demirel, Aridogan, Ozturk and Korkmaz2006) and Australia (Hughes et al., Reference Hughes, Spithill, Smith, Boutlis and Johnson2003). In France, authorized cresspools were also involved in the infection of patients (Gaillet et al., Reference Gaillet, Liance, Rivollet and Houin1983).
In the Guilan endemic province in Iran, villagers collect the aforementioned aromatic freshwater plants and present them beside the streets and in traditional markets throughout the year (Fig. 4A–D). These vegetables are very popular and may be eaten fresh or used to prepare appetizers (Ashrafi, Reference Ashrafi2015).

Fig. 4. Uncontrolled markets of vegetables: (A, B, C) edible wild vegetables sold in the market of Rasht city, Iran; note radishes (B) and watercress (C); (D, E) mobile street selling of wild vegetables in Rasht city, Iran (D) and Nile Delta village, Egypt (E); (F) wild vegetable market in Kutaisi city, Georgia; (G, H) wild vegetable market in Quy Nhon, Vietnam; note radishes (G) and carrots (H). (Photographs S. Mas-Coma).
Non-controlled places where wild plants are sold are usually found in city markets of endemic countries. In Egypt, wild vegetables are sold even individually at the street (Fig. 4E) and uncontrolled wild plant selling is also found in city markets of eastern European countries (Fig. 4F). In Uzbekistan, the relatively high human prevalence in the Samarkand region was related to the important percentage (10.5%) of green vegetables sold in the Samarkand market, which presented encysted metacercariae (Sadykov, Reference Sadykov1988).
In Vietnam, raw vegetables are an important part of the normal diet and wild freshwater plants are easily available in non-controlled city markets (Fig. 4G, H). Water-plants, particularly water-spinaches that are consumed daily by the whole Vietnamese population, are a huge source of infections. A high percentage of trematode contamination in vegetables has been reported in many south-eastern Asian countries (Abdi et al., Reference Abdi, Farhadi, Aghace and Sayehmiri2014; WHO, 2014), especially in Vietnam (Uga et al., Reference Uga, Hoa, Noda, Moji, Cong, Aoki, Rai and Fujimaki2009). There is a strong correlation between infection and travelling, even if it has been demonstrated that contaminated vegetables reach also the big city markets (Ulukanligil et al., Reference Ulukanligil, Seyrek, Aslan, Ozbilge and Atay2001; Fiamma et al., Reference Fiamma, Longoni, Ngo, Phan, Santona, Nu and Paglietti2015). In the Cambodian capital of Phnom Penh, a study showed contamination in water-spinach samples harvested in a lake located at 5–7 km (Vuong et al., Reference Vuong, Nguyen, Klank, Phung and Dalsgaard2007).
Regarding human fascioliasis infection sources through wild vegetables whether directly collected from the field or acquired in urban markets, the recent drive to ‘go green’ as a healthy approach to the modern artificial lifestyle in today developed societies poses evident problems. This recent fashion has shown to underlie an unprecedented increase in the consumption of fresh, raw/green fruit and vegetables (Broglia and Kapel, Reference Broglia and Kapel2011; Hotez et al., Reference Hotez, Alvado, Basáñez, Bolliger, Bourne, Boussinesq, Brooker, Brown, Buckle, Budke, Carabin, Coffeng, Fevre, Fürst, Halasa, Jasrasaria, Johns, Keiser, King, Lozano, Murdoch, O'Hanlon, Pion, Pullan, Ramalah, Roberts, Shepard, Smith, Stolk, Undurraga, Utzinger, Wqng, Murray and Naghavi2014). Unfortunately, this appears to be a real challenge, because this drive appears to be poorly backed by water safety, fertilizer–pesticide use control and waste management. The consumption of poorly monitored, produced and stored fresh green vegetables has contributed to an increased spread of plant/food-borne trematodiases, including fascioliasis among other health problems (Lev and Rager-Zisman, Reference Lev and Rager-Zisman2014). Indeed, individual private garden cultivation of imported Asian vegetables has been suggested to underlie recent fascioliasis epidemic cases in Switzerland (Gottstein, personal communication).
Ingestion of freshwater cultivated plants
Several metacercariae-carrying species may even be so important in the human diet of a given area, as to be man-produced (at family or even industrial level) and commercially sold in public markets, explaining infection of subjects living far away from the endemic area.
Wild watercress is collected and eaten, but it is also cultivated in small family gardens (Fig. 3C, D) and farms. The plant is also produced commercially on large farms and sold in supermarkets, as in Europe and Australia. A study in France showed that home-grown, wild and commercially grown watercress was the cause in 23, 8 and 2 cases, respectively (Chen and Mott, Reference Chen and Mott1990).
If not controlled, a watercress culture in the garden may become contaminated by lymnaeids and Fasciola eggs shed by livestock moving around close to the garden and which may reach the watercress beds by passive transport through rainwater. In given cases, such familial watercress cultures have allowed understanding human infection presenting familial clustering in places which are non-typical for fascioliasis transmission, such as in mountains lacking wide grass fields (Fig. 3C, D) (Gil-Benito et al., Reference Gil-Benito, Mas-Coma, Quilici and Ciolkovitch1991a, Reference Gil-Benito, Ciolkovitch, Mas-Coma and Quilici1991b). Gardens provide an efficient and economic means of vegetable production in the periurban areas. If these actívities are poorly managed and untreated human or animal excreta are used as a fertilizer, the potential for transmission should be monitored.
Unexpected problems of contamination of watercress cultures due to disease spread by an introduced sylvatic reservoir animal as the nutria (Menard et al., Reference Menard, Agoulon, L'Hostis, Rondelaud, Collard and Chauvin2001; Houin et al., Reference Houin, Moquet, Czeher and Vallois2004) appeared related to the emergence of human fascioliasis in concrete areas of France. It was up to this rodent species Myocastor coypus, originally of South America where it already proved to be a good definitive host for the liver fluke (Gayo et al., Reference Gayo, Cuervo, Rosadilla, Birriel, Dell'Oca, Trelles, Cuore and Mera y Sierra2011), to unexpectedly spread F. hepatica eggs in watercress beds made in the way to avoid contamination from ruminants. The aforementioned fascioliasis emergence was described as the first epidemic due to the ingestion of cultivated watercress (Mailles et al., Reference Mailles, Vaillant, Schepens, Aajana, Capek, Ilef, Fillebeen, Lefort, Therouanne, Volant, Flavigny and De Valk2003, Reference Mailles, Capek, Ajana, Schepens, Ilef and Vaillant2006).
In Korea, an aquatic plant known as Water dropwort (Oenanthe javanica) is a perennial herb with a distinctive aroma and is cultivated in marshy areas of Asia and Australia. The fresh stems and leaves are widely used as a salad or as a seasoning in soups and stews in Korea. Water dropwort has also been used in Korea as a folk medicine for the treatment of jaundice, hypertension, fever, abdominal pain, leucorrhea, mumps and urinary difficulty. In a survey, the presence of F. hepatica cox1 and ITS-2 DNA markers were detected in two samples among 500 samples assessed, confirming a 0.4% contamination (Choi et al., Reference Choi, Kim, Quan, Ryu, Sun and Lee2015). The prevalence in this study was lower than that in watercress in France (1.2–2.4% annually) (Dreyfuss et al., Reference Dreyfuss, Vignoles and Rondelaud2005).
Throughout Asia, many of the aforementioned edible vegetables involved in trematode metacercariae transmission such as water caltrop, water chestnut, water lotus, water bamboo, water hyacinth, water lily and water morning glory or water spinach are cultivated in several uncontrolled places to respond to the local demand (Mas-Coma et al., Reference Mas-Coma, Bargues and Valero2005). The water morning glory or water spinach, locally known as ‘rau muong’, is widely cultivated in fields neighbouring villages and even cities where human fascioliasis infection appears to be frequent in Vietnam (Fig. 3E). Sources of Fasciola contamination in agricultural products have been noted to include soil, feces, irrigation water, inadequately composted manure, wild and domestic animals, dirty equipment and human handling (Berger et al., Reference Berger, Sodha, Shaw, Griffin, Pink, Hand and Frankel2010). Differences in prevalence may be induced by various factors such as host distribution, locality and environmental conditions (Choi et al., Reference Choi, Kim, Quan, Ryu, Sun and Lee2015). In southern Taiwan, ponds for the cultivation of the water caltrop Trapa bispinosa inhabited by local lymnaeid snail vector species together with the planorbid Segmentina hemisphaerula are typical close to urban settings (Fig. 3F). Cultures of the floating water lily (Nymphaea sp.) in ponds beside dwellings and presenting lymnaeid snails are usual throughout southern Asia (Fig. 3G, H).
Ingestion of terrestrial wild plants
The survival capacity and relative dryness resistance of metacercariae explain the contamination by consumption of wild terrestrial plants collected in dry or moist habitats but which were submerged in water a few weeks or months before, as in places with temporary water bodies in endemic areas of Iran.
In Andean countries, infection of children has been evoked to occur by putting into mouth, sucking, chewing or even eating terrestrial wild plants, mainly with juicy, succulent stems, which grow in places with frequent freshwater on ground, whether because of their presence at the margins of rivers, streams and lagoons or close to such water bodies giving rise to periodic or sporadic floodings of the surrounding areas in periods of water level rise. This typically occurs after rainfall but may also be related to man-made irrigation strategies. The amphibious characteristics of the lymnaeids, very pronounced in the Galba/Fossaria members and also in several species of the Radix group, underlie the possibility of metacercariae to attach to such vegetables during the periods in which the base of their stems is submerged.
It has been shown that cercariae of Fasciola encyst on objects just under the surface of the water (Hodasi, Reference Hodasi1972; Ueno and Yoshihara, Reference Ueno and Yoshihara1974; Dumag et al., Reference Dumag, Batolos, Escandor, Castilo and Gajudo1976). Hence, this means that metacercariae will be attached to the plant portions which were immersed in water. This explains why the base of the stems but also parts of tubercles protruded over the soil surface, may participate in human and animal infection.
Ingestion of terrestrial cultivated plants
The amphibious characteristics of vector species such as G. truncatula and other Galba/Fossaria species, but also small Asian Radix species, explain the fascioliasis transmission foci in plantations of non-aquatic vegetables needing frequent irrigation.
Rice is a good example of a terrestrial plant which needs plenty of irrigation for its cultures. Indeed, rice fields are ideal habitats for lymnaeid vector development, such as G. truncatula in the Albufera rice fields in Spain (Fig. 5A) (Valero et al., Reference Valero, Marti, Marcos, Robles and Mas-Coma1998), in the wide rice fields inhabited by Radix auricularia in Guilan lowlands, Iran (Fig. 5B), or in those contaminated by R. viridis throughout Vietnam (Fig. 5C). In Egypt, the closeness of rice fields to dwellings of village suburbs is risky for children (Fig. 5D). Rice fields become appropriate for fascioliasis transmission when animal dung or manure (Suhardono et al., Reference Suhardono, Roberts and Copeman2006a) is used for fertilization or culture fields are visited by livestock (Fig. 10F), both aspects being frequent in Asian countries. In its turn, animals may become infected in these rice fields (Ueno and Yoshihara, Reference Ueno and Yoshihara1974) or outside them, as in stall-fed buffaloes by managing the feeding on rice straw (Mahato and Harrison, Reference Mahato and Harrison2005).

Fig. 5. Rice and other cultivated terrestrial plants needing intense irrigation: (A) the loamy soil after rice irrigation is ideal for lymnaeid snails vectors, as in Valencia, Spain; (B) rice fields with Radix auricularia in Guilan lowlands, Iran; (C) irrigated rice fields with Radix viridis surrounding Hanoi, Vietnam; (D) rice fields close to dwellings of village suburbs in the Nile Delta region, Egypt; (E) terrestrial vegetables irrigated by water canals contaminated by lymnaeid snail vectors in the Nile Delta Region, Egypt; (F) khat bush in Ethiopia; note livestock in the background. (A-E: photographs S. Mas-Coma; F: free online at https://es.dreamstime.com/foto-de-archivo-libre-de-regal%C3%ADas-arbustos-con-las-hojas-del-khat-image39802405, accessed 22.2.2018).
In Egypt, many species of non-aquatic vegetables and weeds are eaten raw as salads. These include the garden rocket Eruca sativa (a salad vegetable locally known as ‘El gargeer’), the lettuce Lactuca sativa (known as ‘EI khas’, a leaf vegetable most often used for salads) and the leek Allium porrum (‘EI korrat’). These plants are cultivated along the banks of water channels and need frequent irrigation (Fig. 5E). On collection, they are washed in the nearby water body during their preparation for marketing to have a beautiful green colour. Irrigation and washing expose them to become a carrier of the encysted metacercariae of Fasciola species, thus conveying infection to man (Motawea et al., Reference Motawea, El-Gilany, Massoud, Rizk, El-Shazly and Gaballah2001a).
Other such vegetables on which attached metacercariae have been found in Egypt are the parsley Petroselinum sativum whose edible aromatic leaves are used as a seasoning or garnish and also used in cooking, and the common purslane Portulaca oleracea, an annual succulent that may be eaten as a leaf vegetable used raw in salads (El-Sayed et al., Reference El Sayed, Allam and Osman1997; Motawea et al., Reference Motawea, El-Gilany, Massoud, Rizk, El-Shazly and Gaballah2001a).
Thanks to transport of vegetables, both aquatic and terrestrial, from rural endemic zones to cities, plants carrying metacercariae can be sold in non-controlled city markets giving rise to urban infection, as in Europe, Egypt (Fig. 4E), Bolivia (Mas-Coma et al., Reference Mas-Coma, Angles, Esteban, Bargues, Buchon, Franken and Strauss1999b) or Vietnam (Fig. 4G, H). Metacercariae of F. hepatica were found in 1% of lettuces of a local market in the Mantaro valley, Peru (Bendezu, Reference Bendezu1969). In the Peruvian capital of Lima, among 277 patients infected by F. hepatica, 31.6% mentioned having eaten lettuce, 10.5% alfalfa and 5.3% spinach (Blancas et al., Reference Blancas, Terashima, Maguiña, Lujan, Alvarez and Casanova2004).
In the recent decades, a slow-growing shrub or tree native to the Horn of Africa and the Arabian Peninsula has repeatedly been involved in human fascioliasis infection, namely Catha edulis (Celastraceae), known as khat, qat, gat or Arabian tea (Fig. 5F) (Doherty et al., Reference Doherty, Price, Moody, Wright and Glynn1995; Cats et al., Reference Cats, Scholten, Meuwissen and Kuipers2000; De Bree et al., Reference De Bree, Bodelier and Verburg2013). This plant grows especially well in moist conditions. Therefore, a heavy irrigation of khat cultures starts around a month before they are harvested to make the leaves and stems soft and moist. The fresh leaves or the soft part of the stem are chewed with either chewing gum or fried peanuts to make it easier to chew, in the way to achieve a state of euphoria and stimulation. They are also, but less frequently, dried and consumed as a tea.
Khat chewing is a social tradition since thousands of years among people of countries of the aforementioned region. WHO classified it in 1980 as a drug of abuse that can produce mild to moderate psychological dependence (Nutt et al., Reference Nutt, King and Blakemore2007). Its production, sale and consumption are legal in countries such as Djibouti, Ethiopia, Somalia and Yemen (Chevalier, Reference Chevalier1949). Khat leaves have been chewed by generations in countries of the Horn of Africa, mainly Yemen, for their stimulant properties. Its young fresh leaves are especially valued for their potency. For khat transport, freshly picked leaves are, therefore, usually kept damp and wrapped in banana leaves. In Yemen, khat is so popular that its cultivation requires much of the agricultural resources of the country. Around 40% of the water supply of the country is destinated to its irrigation. Additionally, khat is important in Yemen because it provides a high income for farmers. All this defines a worrying scenario of khat-underlying human fascioliasis in these countries.
Khat is nevertheless a controlled substance in other countries as the USA, Canada and Germany. Traditionally, the use of khat has been confined to regions where it is grown, given that only the fresh leaves offer the desired stimulating effects. However, improved roads, off-road motor vehicles, and air transportation have increased its global distribution in recent years, therefore allowing to understand reports of this plant in the UK, Italy, The Netherlands, Israel, Canada, USA, Australia and New Zealand.
The diagnosis of patients infected in European countries due to the consumption of imported non-controlled khat demonstrates that contaminated cultivated plants in one country may be exported to other countries bearing still viable metacercariae. Indeed, a 45-year-old woman originally from Yemen have not travelled outside the UK was supposed to acquire fascioliasis through chewing khat leaves in London (Doherty et al., Reference Doherty, Price, Moody, Wright and Glynn1995). In The Netherlands, a 36-year-old Somalian man with fascioliasis reported that he had never eaten liver or watercress or other wild water plants but chewed leaves of khat imported from Kenya where khat shrubs were cultivated in areas housing sheep and were irrigated with local water. The survival of the encysted metacercariae was deduced to have been prolonged because the freshly plucked khat leaves were kept damp and wrapped in banana leaves during transport (Cats et al., Reference Cats, Scholten, Meuwissen and Kuipers2000). Also in The Netherlands, another 24-year-old Somalian man infected by Fasciola admitted chewing freshly imported khat leaves, which was most likely the source of infection (De Bree et al., Reference De Bree, Bodelier and Verburg2013). It becomes evident that laws for the control of fascioliasis risks posed by khat exportation are needed.
Ingestion of traditional local dishes made with contaminated sylvatic plants
In the fascioliasis endemic Iranian province of Guilan, there are several very popular kinds of wild aromatic plants, such as species of Eryngium and Mentha, which are (i) eaten raw, (ii) ground and mixed with walnuts, various spices, garlic and fresh olives for the preparation of an appetizer called ‘zeitoon-parvardeh’ (Fig. 6A, B) or (iii) used in the preparation of a paste called ‘delar’ along with a great quantity of salt as a condiment (Fig. 6C, D). The very high quantity of salt used for ‘delar’ preparation is at the base for the local name ‘green salt’ also given to this specialty. This paste may be stored for consumption over several months.

Fig. 6. Traditional culinary specialities made from popular aromatic wild plants, involved in human fascioliasis in the endemic province of Gilan, Iran: (A, B) “Zeitoon-Parvardeh”, a speciality which is made by mixing the grounded local wild plants with other ingredients and fresh olives (A) and used as an appetizer dish (B); (C, D) ”Delar”, a speciality which may be stored for consumption over several months (C) and is served as a traditional herbal paste (D). (A, D: photographs K. Ashrafi; B, C: photographs S. Mas-Coma).
The aromatic vegetables used for these two traditional home-made foods are usually sold throughout the year mainly in the streets of all endemic areas in Guilan Province (Fig. 6A–D). The consumption of these two traditional local foods has been shown to be the main source of human infections in that area. They are also believed to have played an important role in the large outbreak involving around 10 000 people in the Bandar-Anzali and Rasht districts in 1989 and in the subsequent outbreak affecting around 5000 people of the same area of the Guilan province occurred some 10 years later (Ashrafi et al., Reference Ashrafi, Valero, Massoud, Sobhani, Solaymani-Mohammadi, Conde, Khoubbane, Bargues and Mas-Coma2006a, Reference Ashrafi, Valero, Forghan-Parast, Rezaeian, Shahtaheri, Hadiani, Bargues and Mas-Coma2006b). Additionally, these foods represent a risk for fascioliasis spread when Guilan inhabitants give these appetizer and condiment as a present to other family members living in other Iranian provinces.
Ingestion of raw liver
Given experimental results suggested that humans consuming raw liver dishes prepared from fresh livers infected with immature flukes may also become infected, because early migrating flukes present in the ingested infected liver may keep the capacity to re-start the intraorganic migration. In a first experiment, twenty-four mice were inoculated orally, each with a mean number of 68 freshly recovered immature flukes. The livers of 7 of the 24 recipient mice showed migratory lesions of capsular and subcapsular granulomatous infiltration and two of those mice also had haemorrhagic lesions typical of those caused by active migration of early immature flukes (Taira et al., Reference Taira, Yoshifuji and Boray1997). In a second experiment, 10 piglets were given fresh livers of mice harbouring approximately 2000 live immature flukes aged 3–7 days. Granulomatous lesions were found in all pigs at necropsy, except in those that were given livers containing flukes aged 7 days. From the 10 pigs given livers, 65 live flukes were recovered, 0.29% of the estimated number of immature flukes given (Taira et al., Reference Taira, Yoshifuji and Boray1997).
Such an infection source may give rise to confusion with the so-called spurious fascioliasis throughout (Mas-Coma et al., Reference Mas-Coma, Bargues and Valero2014a; Reference Mas-Coma, Agramunt and Valero2014b) and also with spurious paramphistomiasis in Africa.
People ingesting infected domestic animal livers (mainly cattle, sheep, goat and pig) a short time before may reflect ‘false’ fascioliasis when the fluke eggs are found in their stools (Stork et al., Reference Stork, Venables, Jennings, Beesley, Bendezu and Capron1973; Ragab and Farag, Reference Ragab and Farag1978; Campo et al., Reference Campo, Marcen, Gonzalez, Milazzo, Pascual and Ponce1980). Such spurious infection may give rise to false positives. Although an expert microscopist may differentiate the somewhat degenerated aspect of ‘eggs in transit’ from ‘normal eggs’, to avoid confusion in such cases diagnosis requires placing the patient on a liver-free diet and performing repeated follow-up stool examinations.
It should also be considered that paramphistomid flukes are frequently infecting livestock throughout and that their eggs are very similar to those of fasciolids, both in form and size, and may therefore be easily confused when diagnosing animals by coprological analyses. Fortunately, however, paramphistomid flukes do not develop in humans, but this does not exclude the possibility to find ‘paramphistomid eggs in transit’ in human stools shortly after having consumed an animal meat (mainly rumen) infected by paramphistomid flukes, mainly in several parts of Africa.
Six main features allow for the ascription of eggs to Fasciola and differentiate them from the also oval, operculate and non-embryonated eggs from Paramphistomidae:
(a) fasciolid eggs are brownish-yellowish, whereas paramphistomid eggs are clear, transparent or silver-grey;
(b) fasciolid eggs are oval with a common trend to become slender at both ends, whereas paramphistomid eggs are oval with a rectangular trend, i.e. with wide ends;
(c) as a consequence of the aforementioned characteristic, the operculum width/maximum egg width ratio is smaller in fasciolid eggs than in paramphistomid eggs;
(d) in fasciolid eggs the inner contents shows brownish granules smaller in size than the clear cells inside paramphistomid eggs;
(e) at the abopercular end of the shell surface of Fasciola eggs there is often a typical roughened or irregular, more intense brownish dark area which may sometimes appear laterally displaced (Valero et al., Reference Valero, Perez-Crespo, Periago, Khoubbane and Mas-Coma2009), whereas this is absent in paramphistomid eggs.
Moreover, it has been noted that the eggs of these trematodes may be differentiated by contrast stain, eggs of Fasciola showing yellowish colour while eggs of paramphistomids staining when adding a drop of methylene blue or methyl green solution to the sample sediment (Hansen and Perry, Reference Hansen and Perry1994).
In fact, the possibility of acquiring a Fasciola infection by eating an infected animal liver has been suggested since long ago, although not regarding the capacity to give rise to a hepatic infection by the flukes but to a clinical syndrome known as ‘Halzoun’ (i.e., ‘suffocation’), that manifests as an acute allergic edematous reaction involving the upper respiratory tract and nasopharyngeal mucosa. Such a syndrome is known to follow the consumption of raw sheep or goat liver, a food presentation in some countries such as Lebanon, Syria and Iran. Halzoun consists in the temporary attachment to the pharyngeal mucosa of adult worms, which have been ingested along with raw livers of goats and sheep, used for sacrificial purposes and later eaten in religious festivals (Siavashi et al., Reference Siavashi, Assmar and Vatankhah2002). This localized infection produces an edematous congestion of the soft palate, pharynx, larynx, nasal fossae and Eustachian tubes, accompanied by dyspnea, dysphagia, deafness, and, in a few cases, resulting in asphyxiation (Faust, Reference Faust1949).
Different causal agents have been involved in that syndrome (Khalil et al., Reference Khalil, Haddad, Otrock, Jaber and Farra2013). Fasciola hepatica was the first to be described as an agent causing Halzoun. The immature worms of F. hepatica were considered to be the causative agent at that time (Khouri, Reference Khoury1905). Much time later, a similar syndrome referred to as ‘buccopharyngeal distomatosis’ was attributed to F. hepatica or another unknown trematode (Brumpt, Reference Brumpt1936). Since then, similar clinical syndromes, considered to be synonymous to Halzoun syndrome, were described in the literature and were attributed to different pathogens and other parasites with buccopharyngeal tropism (Khalil et al., Reference Khalil, Haddad, Otrock, Jaber and Farra2013). On the basis of clinical cases, Halzoun was attributed to the leech Limnatis nilotica, without ruling out the existence of another form linked to the trematode Clinostomum complanatum (Witenberg, Reference Witenberg1944). Again on the basis of clinical cases, two forms of Halzoun were described, one caused by F. hepatica with the other by L. nilotica (Watson and Kerim, Reference Watson and Kerim1956). When comparing Lebanese Halzoun and the nasopharyngeal linguatuliasis known as Marrara syndrome in Sudan, the larva of the pentastomid Linguatula serrata was also identified as the parasite of Halzoun (Schacher et al., Reference Schacher, Saab, Germanos and Boustany1969; Yagi et al., Reference Yagi, El Bahari, Mohamed, Ahmed, Mustafa, Mahmoud, Saad, Sulaiman and El Hassan1996). More recent publications have attributed Halzoun to accidental infestation by the larvae of L. serrata, including also Greece (Papadakis and Hourmouziadis, Reference Papadakis and Hourmouziadis1958) and Egypt (Morsy et al., Reference Morsy, El-Sharkawy and Lashin1999) Despite their striking resemblance in clinical presentation, few differences exist between these food-borne diseases, i.e. expectoration of worms is rarely observed in the Lebanese Halzoun patients whereas it is a common event in Marrara syndrome (Khalil et al., Reference Khalil, Haddad, Otrock, Jaber and Farra2013). The most recent study on Halzoun, performed in Lebanon, has added a new causal agent, the trematode Dicrocoelium dendriticum (Khalil et al., Reference Khalil, Haddad, Otrock, Jaber and Farra2013). It has moreover suggested that the immature forms of F. hepatica described by A. Khoury as an agent of Halzoun in 1905, may, in fact, have been D. dendriticum, since both trematode species are frequently found together in the liver of ruminants. Hence, this finding indicates the need to verify whether Fasciola worms are really able to cause pharyngeal fascioliasis. Indeed, experimental work confirmed that F. hepatica adults are unable to attach themselves to the mucosa of the pharynx and can be swallowed without symptoms of pharyngeal irritation arising (Watson and Kerim, Reference Watson and Kerim1956), and the experimental use of young F. hepatica failed in the attempt to infect dogs (Brumpt, Reference Brumpt1936). However, the fact that the disease was successfully reproduced in rabbits (Khouri, Reference Khoury1905) keeps the question open about the possibility of Fasciola also being involved in Halzoun after ingestion of raw meat from sheep and goats.
Drinking of contaminated water
The possibility of being infected by means of drinking water carrying floating metacercariae originated from natural water collections inhabited by lymnaeid snail vectors in the field in an endemic area cannot be neglected (Bargues et al., Reference Bargues, Funatsu, Oviedo and Mas-Coma1996).
Indeed, natural freshwater drinking was known to be a potential human infection source according to the anamnesis of many patients in the past (Bürgi, Reference Bürgi1936). Contaminated water has even been mentioned to have been the infection source in collective infections (Sedallian et al., Reference Sedallian, Maral and Perroin1949). However, the infection of the definitive host, whether humans or animals, was traditionally considered secondary or even rare when compared with infection rates by metacercariae attached to vegetables, both in developed countries (Arjona et al., Reference Arjona, Riancho, Aguado, Salesa and Gonzalez-Macias1995) and in developing countries (Mas-Coma et al., Reference Mas-Coma, Angles, Strauss, Esteban, Oviedo and Buchon1995).
In Europe, in an only one patient of the 75 cases reviewed was the antecedent ingestion of watercress not mentioned, and in a series of 18 patients, only four of them reported frequent ingestion of water from unsanitized sources (Arjona et al., Reference Arjona, Riancho, Aguado, Salesa and Gonzalez-Macias1995). In the Northern Bolivian Altiplano, the hyperendemic area with the highest prevalences and intensities known, only in one community was reference made to the potential role of water as a human infection source. In this community, all children surveyed consumed previously untreated water from a well and a canal shared with animals. Both well and canal were noted to be places where humans and animals eliminated biological excreta, thus causing their contamination. Food, mainly local vegetables, were washed with contaminated water, increasing the risk of infection. In this survey, it was concluded that fascioliasis may be acquired by drinking contaminated water instead of eating watercress and other aquatic plants, since the inhabitants mentioned to not usually eat these plants (Mas-Coma et al., Reference Mas-Coma, Angles, Strauss, Esteban, Oviedo and Buchon1995).
The rarity of human infection by water drinking when compared with infection by ingestion of contaminated vegetables had been explained by two facts:
(i) Non-attached metacercariae were proved to sink down to the bottom of the water body quite shortly after cercarial shedding by the snail, due to the progressive impregnation of the metacercarial floaters with different natural water materials giving rise to the increase of the weight of the metacercariae. This was observed in both laboratory (Vareille-Morel et al., Reference Vareille-Morel, Dreyfuss and Rondelaud1993) and natural conditions (Rondelaud et al., Reference Rondelaud, Vignoles, Vareille-Morel, Abrous, Mage, Mouzet and Dreyfuss2004).
(ii) Other results suggested that humans and animals might be infected with Fasciola sp. in endemic areas by drinking the water of small streams or banks contaminated with the metacercariae appearing in the feces (45% have lost their rough gelatinous outer cyst coat, appearing white or cream coloured) shed by lymnaeid snails having previously ingested normal encysted metacercariae (Taylor and Parfitt, Reference Taylor and Parfitt1957; Yoshihara and Ueno, Reference Yoshihara and Ueno2004), although here also the faecal metacercariae, chained inside faecal cylinders, fell to the bottom. These metacercariae shed in snail feces experimentally proved to be similarly infective than normal metacercariae (Yoshihara and Ueno, Reference Yoshihara and Ueno2004).
However, the importance of fascioliasis transmission through water is under reconsideration today after many more recent indirect results suggesting a higher role of water as human infection source (Mas-Coma, Reference Mas-Coma, Cotruvo, Dufour, Rees, Bartram, Carr, Cliver, Craun, Fayer and Gannon2004).
In the French Mediterranean island of Corsica where several human infection cases were reported (Gil Benito et al., Reference Gil-Benito, Ciolkovitch, Mas-Coma and Quilici1991b), water fountains for humans together or beside watering troughs for livestock constructed on the borders of the roads are inhabited by lymnaeid snails and visited by free moving livestock for water drinking, thus representing an evident human infection risk place (Fig. 7A; Gil Benito et al., Reference Gil-Benito, Mas-Coma, Quilici and Ciolkovitch1991a). Similar fountains to be shared by humans and livestock are found in Eastern Europe, as for instance along roads in Georgia (Fig. 7B).

Fig. 7. Drinking water as a human fascioliasis infection source: (A) lymnaeid contaminated on-road fountain including bilateral watering troughs for free moving livestock in Corsica, France; (B) lymnaeid contaminated fountain and watering trough in endemic area of Georgia; (C) collecting water for home in the overflowed water from a broken artificial fountain in the Northern Altiplano of Bolivia; (D) child collecting water for home in canal in the Nile Delta region, Egypt; (E) man-made irrigation system canal on soil inhabited by lymnaeids for the water supply of dwellings in the Peruvian Altipllano; (F) bathing/washing the buffalo in large lateral canal of the Nile river, Egypt. (A, B, D, E, F: photographs S. Mas-Coma; C: photograph J.G. Esteban).
In the Northern Bolivian Altiplano human hyperendemic area, 13% of the metacercariae of all isolates proved experimentally to be floating (Bargues et al., Reference Bargues, Funatsu, Oviedo and Mas-Coma1996). This is worth mentioning owing to the very high number of cercariae-shedding lymnaeids which may be found in that hyperendemic area, e.g. 31.6% prevalence in lymnaeids from the locality of Tambillo. Up to seven metacercariae in only half a liter of water from the small river crossing Tambillo were found (Mas-Coma, Reference Mas-Coma, Cotruvo, Dufour, Rees, Bartram, Carr, Cliver, Craun, Fayer and Gannon2004; Bargues and Mas-Coma, unpublished). In the Bolivian Altiplano, moreover, people collect water to home from overflowed waters from broken man-made fountains, these overflowed waters presenting lymnaeid snail vectors (Fig. 7C).
Similarly appears to happen in high altitude Andean valleys of Peru, where for instance a high prevalence of 20.5% was detected among 462 lymnaeids collected in front of the school of Santa Rosa de Chaquil, Cajamarca province (Bargues et al., Reference Bargues, Artigas, Khoubbane, Ortiz, Naquira and Mas-Coma2012), a locality in which 47.7% of the children were infected (Gonzalez et al., Reference Gonzalez, Esteban, Bargues, Valero, Ortiz, Naquira and Mas-Coma2011). Also in Peru, in a series of 277 patients with fascioliasis diagnosed in Lima, a 10.5% mentioned having drunk water from ‘puquiales’ (natural water from small streams) (Blancas et al., Reference Blancas, Terashima, Maguiña, Lujan, Alvarez and Casanova2004).
Human infection by water drinking is also suggested by the statistically significant positive associations between liver fluke infection and infection by protozoans and other helminths which are waterborne. Thus, F. hepatica infection appears associated to Giardia intestinalis infection in the Northern Altiplano of Bolivia (Esteban et al., Reference Esteban, Flores, Aguirre, Strauss, Angles and Mas-Coma1997a) and Peru (Esteban et al., Reference Esteban, Gonzalez, Bargues, Angles, Sanchez, Naquira and Mas-Coma2002), to Entamoeba hartmanni in Cajamarca, Peru, where G. intestinalis also shows high prevalences paralleling those very high fascioliasis prevalences (Gonzalez et al., Reference Gonzalez, Esteban, Bargues, Valero, Ortiz, Naquira and Mas-Coma2011), and to Entamoeba coli, Chilomastix mesnili and Schistosoma mansoni in the Nile Delta, Egypt (Fig. 7D; Esteban et al., Reference Esteban, Gonzalez, Curtale, Muñoz-Antoli, Valero, Bargues, El Sayed, El Wakeel, Abdel-Wahab, Montresor, Engels, Savioli and Mas-Coma2003).
In many human hyperendemic areas of the Americas, people do not have a history of eating watercress (Hillyer and Apt, Reference Hillyer and Apt1997). Of particular interest in that sense are the reports on human infection in artificial irrigation areas, even giving rise to human fascioliasis endemic situations. In the Peruvian Altiplano, the human endemic area of Asillo is a man-made irrigation area where human inhabitants do not have the tradition to consume freshwater plants but to collect water for drinking from the irrigation canals inhabited by the snail vector G. truncatula (Fig. 7E; Esteban et al., Reference Esteban, Gonzalez, Bargues, Angles, Sanchez, Naquira and Mas-Coma2002).
In Argentina, several outbreaks appear related to visits of recreational field areas by thousands of tourists and campers during weekends. These areas include natural water collections inhabited by lymnaeid vectors and frequented by Fasciola-infected livestock (Mera y Sierra et al., Reference Mera y Sierra, Agramunt, Cuervo and Mas-Coma2011). The increasing infection risk posed by recreational waters due to the impact of climate change is undoubtedly a future challenge (de Roda Husman and Schets, Reference de Roda Husman and Schets2010).
In the Nile Delta region, persons living in houses where piped water is present showed to have a higher infection risk (Curtale et al., Reference Curtale, Mas-Coma, Hassanein, Barduagni, Pezzotti and Savioli2003). In Egypt, children have a risk to become infected when washing the livestock in the waters of the large irrigation canals and also when drinking water from the smaller irrigation canals bordering the plantations surrounding the outer village suburbs, the lymnaeid vectors having colonized all such canals (Bargues et al., unpublished).
The importance of water as human infection source is also proved by the high prevalence reduction effectivity obtained in an assay performed by the Egyptian Ministry of Health and Population, the Italian Cooperation and the WHO Collaborating Centre of Valencia in an endemic village of the Nile Delta human hyperendemic area with the construction of the so-called ‘washing units’ (Curtale et al., unpublished). In these ‘washing units’, water from a big canal was pipped and filtered by means of a swimming pool filtration equipment before flowing up to the taps of each one of several laundries. Women of this village were a successfully convinced to daily use such installation as a source for (i) water drinking, (ii) collecting water for food preparation and washing and (iii) water for washing of kitchen utensils and dresses (Fig. 8A–D). The drastic human prevalence reduction obtained, from a previous 18% (Esteban et al., Reference Esteban, Gonzalez, Curtale, Muñoz-Antoli, Valero, Bargues, El Sayed, El Wakeel, Abdel-Wahab, Montresor, Engels, Savioli and Mas-Coma2003) to a subsequent 2% after the construction and use of these ‘washing units’ (Curtale et al., unpublished), suggested human infection in this village to be mainly related to water drinking (Mas-Coma, Reference Mas-Coma, Cotruvo, Dufour, Rees, Bartram, Carr, Cliver, Craun, Fayer and Gannon2004).

Fig. 8. The "Washing Units" control pilot intervention implemented in the hyperendemic village of Tiba, Delengat, Nile Delta, Egypt: (A) building with several laundries where filtered water flows through the taps; (B, C) women of this village were successfully convinced to daily use such installation as a source for water drinking and collecting water to home for food preparation (B), as well as for washing of kitchen utensils (B) and dresses (C); (D) water from a big canal was pipped and filtered by means of a swimming pool filtration equipment. (Photographs S. Mas-Coma).
Anyway, this result indicating the importance of water as human infection source in a village does not mean that the sources are the same in other villages, not even in the same endemic area. The epidemiological heterogeneity of human fascioliasis is also evident in that aspect. In the Northern Altiplano, vegetables play a more important role than water drinking in the Bolivian part eastward from Lake Titicaca (Mas-Coma et al., Reference Mas-Coma, Angles, Strauss, Esteban, Oviedo and Buchon1995), whereas water drinking appears to be the almost only human infection source on the Peruvian side of the Lake (Esteban et al., Reference Esteban, Gonzalez, Bargues, Angles, Sanchez, Naquira and Mas-Coma2002). Opposite to that, vegetables and mainly watercress underlie human infection in Europe (Arjona et al., Reference Arjona, Riancho, Aguado, Salesa and Gonzalez-Macias1995).
Cercariae of F. gigantica can also encyst on very small floating particles or on the water surface, in which case they may be swallowed in contaminated drinking water (Mas-Coma and Bargues, Reference Mas-Coma and Bargues1997). Thus, in enzootic areas of F. gigantica as in Africa, contraction of the infection by the animals and their contamination of the area with eggs of the parasite in the feces take place when the animals go to drink (Fig. 7F), rather than when they are grazing in the pasture. Accordingly, avoiding the watering of the animals from swampy banks of rivers and from bodies of water rich in vegetation would considerably reduce the chances of infection.
Drinking of beverages and juices made from local plants
Local beverages and juices have been linked to human infection by Fasciola according to the results of the patient's anamnesis or surveys about risky foods and drinks. Contamination of such beverages and juices may be whether from the plants or the natural water used for their washing and beverage production.
In Andean countries, beverages and juices are produced from leaves of plants and traditionally consumed in mainly Peru, but also Bolivia, Ecuador and Colombia. In Peru, warm beverages called ‘emolientes’ (emollients) were introduced during the colonization period and became so popular as to be sold in every street corner of the capital Lima. These emollients are aqueous drinks made from various medicinal plants, mainly alfalfa and watercress, and are supposed to be good for liver diseases among other illnesses.
These emollients are believed to also play a role in rural areas of Peru, according to their regular appearance among the risk factors deduced from results of questionnaire surveys in endemic areas (Blancas et al., Reference Blancas, Terashima, Maguiña, Lujan, Alvarez and Casanova2004; Raymundo et al., Reference Raymundo, Maco Flores, Terashima, Samalvides, Miranda, Tantalean, Espinoza and Gotuzzo2004; Valencia et al., Reference Valencia, Pariona, Huamán, Miranda, Quintanilla and Gonzales2005; Marcos et al., Reference Marcos, Maco, Samalvides, Terashima, Espinoza and Gotuzzo2006). In a hospital in Lima, a 5.3% among a total of 277 Fasciola-infected patients mentioned to be consumers of emollients (Blancas et al., Reference Blancas, Terashima, Maguiña, Lujan, Alvarez and Casanova2004).
When surveying children in the endemic rural areas of Peru, drinking alfalfa juice appeared as the main exposure factor for F. hepatica infection (Raymundo et al., Reference Raymundo, Maco Flores, Terashima, Samalvides, Miranda, Tantalean, Espinoza and Gotuzzo2004; Marcos et al., Reference Marcos, Maco, Samalvides, Terashima, Espinoza and Gotuzzo2006). A similar result indicating the importance of alfalfa juice in fascioliasis infection was obtained in a survey of schoolchildren in Puebla, Mexico (Zumaquero-Rios et al., Reference Zumaquero-Rios, Sarracent-Perez, Rojas-Garcia, Rojas-Rivero, Martinez-Tovilla, Valero and Mas-Coma2013). Alfalfa (Medicago sativa) is a perennial plant which is cultivated as livestock fodder since the antiquity. Alfalfa has an extremely high nutritive value and it is used in traditional medicine due to its high content in protein, calcium, vitamins, enzymes and amino acids (Amraie et al., Reference Amraie, Farsani, Sadeghi, Khsan, Babadi and Adavi2015). This is why its sprouts are a common ingredient in dishes made in South Indian cuisine. The correlation of alfalfa juice with fascioliasis in children led to suggest that human fascioliasis in Peru should be suspected in patients who have a history of consumption of alfalfa juice when they come from livestock-rearing areas and present with jaundice and eosinophilia (Marcos et al., Reference Marcos, Maco, Samalvides, Terashima, Espinoza and Gotuzzo2006).
The Cape Verde Republic is an Atlantic archipelago in which human fascioliasis has repeatedly been reported. Although most of the patients refer to having consumed watercress (see e.g. Nozais et al., Reference Nozais, Thomas, Bricaire, Danis and Gentilini1998), the possibility for the traditional beverages to be also involved in the transmission to humans should not be neglected (Mas-Coma, Reference Mas-Coma, Cotruvo, Dufour, Rees, Bartram, Carr, Cliver, Craun, Fayer and Gannon2004). One of the most important aspects of Cape Verdean culture is beverage production, for which sugar cane is used on the islands of Santiago and Santo Antão. Indeed, sugar cane grown in swampy areas has already been emphasized as a potential source of metacercarial ingestion in Africa (Speckhart, Reference Speckhart1969).
Ingestion of dishes and soups made with contaminated water
Water containing metacercariae may also contaminate food. Liver fluke infection by ingestion of salads contaminated with metacercariae-carrying water has been reported in Basse-Normandie, France (Cadel et al., Reference Cadel, Barbier, Duhamel and Georges1996).
In the human hyperendemic area of the Northern Altiplano of Bolivia, edible algae as cochayuyo or ‘llayta’ (P. purpurea – Chlorophyta) and similar vegetables as gelatinous species of Nostoc (Cyanobacteria) (Mas-Coma et al., Reference Mas-Coma, Angles, Strauss, Esteban, Oviedo and Buchon1995) present in water collections show sometimes lymnaeid snail specimens moving on their surface, indicating the risk of presenting attached metacercariae. These vegetables are locally used to make soups and frequently appear in questionnaire surveys correlating with infection in children.
Washing of vegetables, fruits, tubercles, kitchen utensils or other objects with contaminated water
Natural water may be an indirect source of fortuitous infection when containing infective metacercariae and contaminating vegetable foods, kitchen utensils and other objects by two ways: whether by washing or when water is used to soak vegetables, fruits or tubercles and utensils. This is an additional way to understand the contamination of terrestrial vegetables (Fig. 9A, B) and may be the only to tell how aerial fruits and subterraneous tubercles may become contaminated by fasciolid metacercariae. Several studies have confirmed that vegetables are good vectors for the transmission of some parasitic diseases in different countries due to the use of contaminated water to irrigate or more often to clean the vegetables (de Oliviera and Germano, Reference de Oliviera and Germano1992; Eraky et al., Reference Eraky, Rashed, Nasr Mel, El-Hamshary and Salah El-Ghannam2014). Indeed, fecal contamination of vegetables and fruits during cultivation and processing for the market is well known since long ago (Geldreich and Bordner, Reference Geldreich and Bordner1971).

Fig. 9. Washing or soaking with contaminated waters: (A) lymnaeid snail vectors on loamy soil surrounding lettuce leaves in Hong Kong; (B) terrestrial wild vegetables collected from wet habitats and washed before consumption in Talesh mountains, Guilan, Iran; (C) housewives and children washing in water canal inhabited by lymnaeid snails and frequented by livestock for drinking, in Atlixco, Puebla State, Mexico; (D) carrots freshen and soaked in stream waters for subsequent marketing in Apartaderos, Merida State, Venezuela; (E, F) housewives and children in traditional washing at river presenting lymnaeid snails and frequented by livestock for drinking, in Mantaro valley, Peru; (G) women washing dresses and utensils in large canals in the Nile Delta region, Egypt; (H) small stream (marked by shrubs and trees linearly arranged from left to right) inhabited by Galba truncatula vectors separating tourist camping (in the background) and football field used by livestock for grazing, in the Mediterranean Corsica island, France. (Photographs S. Mas-Coma).
A curious case was that of a girl who became infected with Fasciola when frequently munching apples without previously peeling them (Ehlers and Knüttgen, Reference Ehlers and Knüttgen1949). This girl collected these apples after falling down into the stagnant water of a canal just beside where she was playing and a canal which was later verified to be inhabited by G. truncatula (Minning and Vogel, Reference Minning and Vogel1950). This case speaks about the opportunistic way of transmission Fasciola may take advantage of.
Of particular interest is the possibility for tubercles to play a role as human fascioliasis infection source. An association between child fascioliasis and the habit of eating raw vegetables was identified in Mexico. The link of fascioliasis risk with consumption of raw vegetables other than watercress should be highlighted, as it suggests contamination when washing terrestrial vegetables with untreated water and/or in plant cultures using natural water for irrigation (Zumaquero-Rios et al., Reference Zumaquero-Rios, Sarracent-Perez, Rojas-Garcia, Rojas-Rivero, Martinez-Tovilla, Valero and Mas-Coma2013). Radish appeared with an 82% among the questionnaires fulfilled by parents of children infected with F. hepatica in a survey of schoolchildren in Puebla, Mexico. In the same survey, terrestrial plants as lettuce, broccoli and spinach appeared however with pronouncedly lower percentages (34, 12 and 8%, respectively) (Zumaquero-Rios et al., Reference Zumaquero-Rios, Sarracent-Perez, Rojas-Garcia, Rojas-Rivero, Martinez-Tovilla, Valero and Mas-Coma2013). In this human endemic area of Puebla, there is the tradition for women and children to wash at small rivers where lymnaeid vectors are present and livestock move around (Fig. 9C). The consumption of radishes has also appeared highlighted as an important risk factor for child infection in a questionnaire survey recently made in Baños del Inca, Cajamarca, Peru (Rodriguez et al., Reference Rodríguez, Rivera, Del Valle, Cerna, Hoban, Chilón and Ortiz2018).
In Venezuela, in Apartaderos, in the endemic high altitude area of Merida, carrots are soaked or freshen in waters of natural streams (Fig. 9D) (Mas-Coma et al., unpublished). In Peru, a similar behaviour of women and children washing in rivers with lymnaeids and livestock is observed in the human hyperendemic area of the Mantaro valley (Fig. 9E, F).
In the hyperendemic area of Cajamarca, Peru, it is a common practice for young children to help their parents in agricultural activities and in the tending of animals. Thus adults and children, whilst performing these activities, stay away from home for many hours. This leads them to drink freshwater and to eat vegetables such as watercress and ‘chocho’ or ‘tarwi’ (Lupinus mutabilis). The latter is an annual plant, a species of lupin grown in the Andes mainly for its edible bean. Lupin seeds are soaked with waters of streams and small rivers of the endemic area and that may therefore present lymnaeid vectors and consequently become contaminated with metacercariae of F. hepatica (Ortiz et al., Reference Ortiz, Cabrera, Jave, Claxton and Williams2000). ‘Chocho’ is also sold in the market of Cajamarca city, thus allowing for urban infection. When asking mothers of the rural endemic area of Cajamarca about risky activities at home, 74% mentioned to consume ‘chocho’ and 39% to drink emollients (Rivera-Jacinto et al., Reference Rivera-Jacinto, Rodríguez-Ulloa, Rojas-Huamán, Valdivia-Meléndez and Saucedo-Duran2010).
In the city of La Paz, just bordering the human fascioliasis hyperendemic area of the Northern Bolivian Altiplano, a high contamination by protozoan and helminth enteroparasites was found in vegetables sold in the city markets, including F. hepatica contaminating a 3.6% of carrots and 17% of watercress (Muñoz Ortiz and Laura, Reference Muñoz Ortiz and Laura2008).
In the human fascioliasis hyperendemic area of the Nile Delta region, Egypt, a possible reason for the high prevalence is thought to be the habit of farmers of picking vegetables and then leaving them immersed in the canals to keep them fresh while they continue picking (Hotez et al., Reference Hotez, Savioli and Fenwick2012). Additionally, in the marketplace as well as in the home, contaminated water containing metacercariae may be used to freshen vegetables, particularly leafy vegetables.
In the same hyperendemic region of Egypt, the presence of farm animals and their sheds inside the houses of their farmers’ owners has been highlighted to be a risk factor for them (Hussein et al., Reference Hussein, El-Din, Mohamad, Mahmoud, Abou Zeid and Khalil2000). These Egyptian farmers living indoors with animals sheds are at high risk of fascioliasis infection (Motawea et al., Reference Motawea, El-Gilany, Massoud, Rizk, El-Shazly and Gaballah2001a). Most of these farmers, their housewives and children wash the animals in canals together with the vegetables, dresses and utensils (Fig. 9G). Afterward, they eat such contaminated vegetables and drink water in such contaminated utensils. During animal washing, their children swim in water and may involuntarily swallow such contaminated water. In such circumstances, the risk of infection with Fasciola species has been observed to be higher in situations of low housing score, little or very low social score, and large family size (Hussein et al., Reference Hussein, El-Din, Mohamad, Mahmoud, Abou Zeid and Khalil2000; Motawea et al., Reference Motawea, El-Gilany, Gaballah, Emara and El Shazly2001b). Anyway, another study reported that there was no significant association between the social class of the family and the increased risk of infection by Fasciola (El-Sahn et al., Reference El Sahn, Farghaly, El-Masry, Mandil, Mohammad and El-Morshedy1995).
Also in the Nile Delta of Egypt, the most exhaustive study on the relationships of the dietary habits and household characteristics with human infection by Fasciola so far made in a human fascioliasis hyperendemic area was carried out (Curtale et al., Reference Curtale, Mas-Coma, Hassanein, Barduagni, Pezzotti and Savioli2003). The habit to consume daily raw seeds was confirmed as an important risk factor (OR = 8.6), followed by the presence of piped water in the latrine (OR = 6.9), the habit to bring the animals to the canal for drinking and/or bathing them (OR = 3.2), and the use to cultivate the vegetables eaten in the household (OR = 3.1) (Curtale et al., Reference Curtale, Mas-Coma, Hassanein, Barduagni, Pezzotti and Savioli2003).
Among the factors investigated, only the presence of cows, buffaloes and/or goats in the household, and the habit to bring those animals to the canal for bathing was significantly associated with the infection. The possibility that people use the irrigation canal to wash dishes, clothes, vegetables and even themselves, close to where domestic ruminants are taken to bath and where also the lymnaeid snail host is present, represents an obvious link between the animal and human infections implying the importance of controlling the animal reservoir to the benefit of humans (Curtale et al., Reference Curtale, Mas-Coma, Hassanein, Barduagni, Pezzotti and Savioli2003). In the French Corsica island, human infection was reported to occur in touristic campings where washing kitchen and table-eating utensils were made in lateral stream inhabited by G. truncatula snails and frequented by cattle for drinking (Fig. 9H).
Two more risk factors, apparently not significant in the univariate analysis, emerged as closely associated with the infection in the logistic regression analysis, namely the direct production of the vegetables eaten in the household and the presence of piped water. The habit of eating raw seeds emerged from the analyses as significantly more frequent among cases than controls. This finding should be interpreted taking into account that those seeds are usually washed several times in the canal before being dried. The washing process makes it possible that metacercaria encyst on the skin of the seeds, and then resist to the drying process, being ingested by the consumer breaking the skins with his teeth (Curtale et al., Reference Curtale, Mas-Coma, Hassanein, Barduagni, Pezzotti and Savioli2003).
However, while it is easy to assume that water used for irrigation is derived from the same canal where animals are bathing, it is more difficult to interpret the association between piped water in the household and infection. The presence of piped water in the household has always been considered an essential element for the control of intestinal parasites. However, the possibility that water sources may be polluted with encysted metacercaria cannot be excluded and should be verified in further studies (Curtale et al., Reference Curtale, Mas-Coma, Hassanein, Barduagni, Pezzotti and Savioli2003).
In the same sense indicates the finding of the lymnaeid vector G. truncatula living on a somewhat muddy sheet over the water border of a geosynthetic waterproof ground of the large artificial water reservoir at the origin of the sewage network for the water supply of a city in Corsica Island (Oviedo et al., Reference Oviedo, Mas-Coma, Dominici and Roche1992).
Methods to assess human infection sources
Assessing Fasciola infection sources for humans is not easy at all. There are different ways to establish which consumable products are susceptible to carry infective metacercariae in a given area:
• analysis of consumable products for the detection of Fasciola metacercariae;
• analysis of the answers to appropriate questions posed to in-hospital-diagnosed infected patients during anamnesis;
• analysis of the answers obtained in adequately oriented questionnaires distributed in surveys performed in human endemic areas.
Detection of metacercariae attached to plants or floating in freshwater
The first of the aforementioned methods is the only way to verify whether a product consumable by humans is able to carry Fasciola metacercariae and consequently a true human infection source. Several aspects should be considered before launching such a study:
• To avoid losing plenty of time analysing numerous materials without finding any metacercariae, the vegetables and water to be analysed should be collected besides or close to places where the lymnaeid vectors are present. Moreover, the collection should be performed whenever possible in the period or shortly after the season during which lymnaeid are shedding the cercariae. Therefore, the seasonality of the transmission in the endemic area in question should be taken into account.
• It should be considered that lymnaeids but also other freshwaters snails belonging to other molluscan groups sharing the same water collections and usually coexisting with lymnaeids, such as physids and planorbids, transmit digenean species of many different trematode groups and that give rise to very similar metacercarial cysts which also attach to the same vegetables. Consequently, the belonging to Fasciola of the metacercariae found should be verified. For such a purpose, there are two ways:
◦ experimentally, by orally infecting an animal model in the laboratory and afterwards obtaining the fasciolid adult by dissection of the animal once appearance of fasciolid eggs verified in the animal stools; the fasciolid adult obtained can be subsequently phenotypically and genotypically characterized for species ascription and even subspecific characterization; this method requires a long time because of the need to complete the prepatent period (from infection up to egg production), but presents the advantage that it moreover furnishes information about the capacity of the metacercariae to give rise to viable adult stages;
◦ molecularly, by means of any of the numerous molecular methods which allow for fasciolid distinguishing, among which methods for DNA sequencing allow for the detection of single-nucleotide polymorphisms and in that way not only differentiate fasciolids from other trematode groups, but also to differentiate between F. hepatica and F. gigantica and even to characterize Fasciola hybrids when in areas of overlap of the two species in Africa and Asia (Mas-Coma et al., Reference Mas-Coma, Valero and Bargues2009a). However, this method does not furnish information about the metacercarial viability nor on their capacity of definitive host infection.
These methods for the detection of metacercariae on plants or in water may be applied whether (i) in the field in an endemic area, (ii) surveillance of vegetables in markets or (iii) to the monitoring of cultures of edible and marketable plants susceptible to become contaminated by lymnaeid snail vectors and feces of infected animals, mainly livestock.
Given the importance of rice fields in fascioliasis transmission (Valero et al., Reference Valero, Marti, Marcos, Robles and Mas-Coma1998), a specific method called ‘Metacercaria Detecting Buoy (MDB) Method’ was developed for the estimation of the contamination of rice fields with Fasciola metacercariae (Ueno et al., Reference Ueno, Yoshihara, Sonobe and Morioka1975; Ueno, Reference Ueno1976). This method is based on the fact that the F. gigantica cyst formation occurs more abundantly in the shallow layer below the water surface, particularly in a range of 2–3 cm deep, and is also apt to occur on materials with a smooth surface (Ueno and Yoshihara, Reference Ueno and Yoshihara1974). A buoy comprises a disk made of foam styrol (10 cm of diameter × 3 cm thick), a polyethylene sheet (16 cm of diameter), a long bolt with a hole at the tip, rubber band, nylon thread (about 35 cm long) and a lead anchor. The bottom and side of the disk are wrapped with a polyethylene sheet, which is fixed to the disk by rubber bands. A nylon thread which has a lead anchor is connected to the tip of the bolt penetrating the disk. One such buoy is installed at each of four corners of a rice field, and numbers of cysted metacercariae counted on each buoy are averaged. The period of stallation depends on the study objective. For a detailed examination of cercarial emergence during the whole growing season of rice, the period covers about 3 months of a whole irrigation period, starting from immediately after transplanting of rice to the end of irrigation. During that period, the polyethylene sheet is replaced every one week. However, if the purpose is simply to know the occurrence and an approximate number of cercariae, it is enough to install 4 buoys for a week at the end of the irrigation period (Ueno, Reference Ueno1976).
To avoid the confusion with metacercariae of other kinds of trematodes, a dissection microscope should be used. The distinction between metacercariae of Fasciola and those of paramphistomid flukes of ruminants which often encyst on the polyethylene sheet should be made. This can easily be done by considering that Fasciola metacercariae are large, light brown coloured, lack black spots and present an outer cyst. On their turn, paramphistomid metacercariae are small, dark brown to black in colour, present black spots and lack an outer cyst.
This method may be useful to assess the seasonality of the infection risk and also to monitor the impact of the use of cattle manure in the rice fields.
Anamnesis in individual patients
Appropriate questions posed to a fascioliasis patient may furnish various information about the infection source. Therefore, it should be taken into account that, when dealing with patients in whom the lack of fasciolid eggs in stools suggests that the disease is still in the invasive or acute phase, the onset of symptoms occurs in that phase shortly after the infection. Patients shedding eggs in stools indicate that they are already in the biliary or chronic phase and, in these cases, one should consider that the previous invasive or acute phase lasts 3–4 months in humans in F. hepatica infection and 1–2 weeks longer when in F. gigantica infection (Valero et al., Reference Valero, Bargues, Khoubbane, Artigas, Quesada, Berinde, Ubeira, Mezo, Hernandez, Agramunt and Mas-Coma2016).
Questionnaire surveys in human endemic areas
Whereas watercress and only a few additional consumption vegetables are the only noted to be involved in Europe, many other vegetables are involved in endemic areas of Latin America, Africa and Asia, and they may additionally be very different in high altitude areas from those in the lowland areas. Unfortunately, looking for metacercariae attached to plants to assess which consumption vegetables are involved in human infection becomes very tedious, long-time consuming and complicated due to the need to experimentally or molecularly verify whether metacercariae belong to Fasciola or to any other of the very numerous trematodes which also give rise to metacercariae attached to freshwater plants. Therefore, an indirect way by using survey questionnaires is usually implemented to get an idea of which consumption vegetables are more risky. Such surveys offer moreover the way to assess the vegetable diet of the local people, which may be very different in one place, area or country from another. In such questionnaires, it should be considered that liver fluke infection may not only be linked to:
(a) different freshwater plants, but also to
(b) terrestrial plants needing considerable irrigation,
(c) other plants which may grow in areas which become flooded during year periods,
(d) sylvatic plants used for the obtaining of local beverages and juices,
(e) fruits growing in aerial parts of bushes and trees and tubercles growing inside the soil but which are usually washed with natural water in small rivers or streams whose waters may have swimming cercariae shed by lymnaeids, and also
(f) plants which are not included in the local people diet but may be sucked or chewed by children when playing outside in the field, or
(g) plants used by adult people for other purposes such as for instance khat in parts of Africa.
When dealing with children, it should be considered that they usually do not distinguish between different vegetable species nor know how their mothers have prepared the vegetable dish. Moreover, the infection may have taken place not by eating a vegetable but by only swallowing, sucking, chewing or stripping it with the teeth when in the field. Asking parents furnishes more believable results. Mothers should be asked not only about vegetables they consume but also on how (and where) they wash (which is the origin of the water they use for it) and prepare them (Fig. 10A). Drinking water source should also be asked for, whether in the field or at home. Asking for the recreational waters visited may be of interest in given areas, as for instance in Argentina (Mera y Sierra et al., Reference Mera y Sierra, Agramunt, Cuervo and Mas-Coma2011). Quantitative correlations with human infection may be assessed by considering that human infection uses to concentrate in families (Fig. 10B) and according to the typically patchy distribution of this waterborne disease linked to water collections inhabited by lymnaeid snail vectors and, in the animal endemic areas but also in the human endemic areas, to the presence of livestock which is usually infected (Mas-Coma et al., Reference Mas-Coma, Angles, Esteban, Bargues, Buchon, Franken and Strauss1999b).

Fig. 10. Surveying and prevention aspects: (A) surveying mothers regarding diet, behaviour, habits and social aspects linked to fascioliasis transmission furnishes results more reliable than surveying children, as made in schools of Cajamarca province, Peru; (B) familiar clustering is usual in fascioliasis, due to the sharing of food and water drinking from the same origin, as in the Nile Delta villages, Egypt; (C) children use to take advantage for risky swimming when accompanying animals to drink or wash in canals, as in the Dominican Republic; (D, E) in the Aymara communities of the Northern Bolivian Altipano, there is the tradition of children (D) and women (E) to be in charge for livestock in the field; (F) allowing livestock to move around or in vegetable cultures facilitates liver fluke transmission when in irrigated fields inhabited by lymnaeid snail vectors, as in Vietnam. (Photographs S. Mas-Coma).
Several such questionnaire surveys have furnished useful information when implemented in different human fascioliasis endemic areas where children are mainly affected by the disease: in the Northern Bolivian Altiplano (Mas-Coma et al., Reference Mas-Coma, Angles, Strauss, Esteban, Oviedo and Buchon1995); in the Nile Delta, Egypt (Curtale et al., Reference Curtale, Mas-Coma, Hassanein, Barduagni, Pezzotti and Savioli2003); in the Mantaro valley, Peru (Marcos et al., Reference Marcos, Maco, Terashima, Samalvides, Espinoza and Gotuzzo2004); in Asillo, Northern Peruvian Altiplano (Marcos et al., Reference Marcos, Maco, Samalvides, Terashima, Espinoza and Gotuzzo2006); in Puebla, Mexico (Zumaquero-Rios et al., Reference Zumaquero-Rios, Sarracent-Perez, Rojas-Garcia, Rojas-Rivero, Martinez-Tovilla, Valero and Mas-Coma2013); and finally, based on the methodology applied in this Mexican survey, also in Baños del Inca, Cajamarca, Peru (Rodríguez et al.,.Reference Rodríguez, Rivera, Del Valle, Cerna, Hoban, Chilón and Ortiz2018).
Dietary habits of the human populations are very important in fascioliasis. Watercress and other aquatic vegetables able to carry attached metacercariae and included in the human diet in different countries serve as vehicles of the infection. The habits of eating raw watercress and other vegetables cause the metacercariae to enter the human alimentary tract, but the possibility of being infected by means of drinking water carrying floating metacercariae should not be overlooked (Bargues et al., Reference Bargues, Funatsu, Oviedo and Mas-Coma1996). In some countries, such as in China, where vegetables are always cooked for eating, the infection may rarely occur by ingestion of unboiled drinking water, or from the metacercariae on cutting boards and other kitchen utensils (Chen and Mott, Reference Chen and Mott1990).
In a deep study on the relation of the diet and household characteristics with Fasciola infection in humans in the Nile Delta of Egypt, factors showing importance were daily raw seed consumption, presence of piped water in the latrine, the habit to bring the animals to the canal for drinking and/or bathing/washing them, and the use to cultivate the vegetables eaten in the household. The presence of cows, buffaloes and/or goats in the household, and the habit to bring those animals to the canal and take advantage for bathing (Fig. 10C), were significantly associated with human fascioliasis. The direct production of the vegetables eaten in the household and the presence of piped water also showed close association (Curtale et al., Reference Curtale, Mas-Coma, Hassanein, Barduagni, Pezzotti and Savioli2003).
In Puebla, Mexico (Zumaquero-Rios et al., Reference Zumaquero-Rios, Sarracent-Perez, Rojas-Garcia, Rojas-Rivero, Martinez-Tovilla, Valero and Mas-Coma2013), the analysis of questionnaire responses showed that, after watercress, fascioliasis risk appeared linked to several terrestrial vegetables which are usually eaten raw, such as radish, lettuce, corncob and spinach after washing them with contaminated water or because of being cultivated using natural water for irrigation in places where water collections are inhabited by lymnaeids.
In another survey, in Peru, in fascioliasis patients diagnosed in an hospital in Lima, only 45.6% mentioned having eaten watercress, the rest having acquired the disease from eating other plants such as lettuce (31.6%), alfalfa (10.5%), or spinach (5.3%), drinking water from the so-called ‘puquiales’ ( = natural water from small streams) (10.5%), or ‘emollients’, a warm beverage made from various plants, chiefly alfalfa (a forage crop introduced by Spanish colonizers into the Americas as fodder for their horses in the 16th century) (5.3%) (Blancas et al., Reference Blancas, Terashima, Maguiña, Lujan, Alvarez and Casanova2004). Alfalfa juice was also found to be a fascioliasis risk factor for children in the human endemic area of the Peruvian Mantaro valley (Marcos et al., Reference Marcos, Maco, Terashima, Samalvides, Espinoza and Gotuzzo2004).
Infectivity of metacercariae
Survival and infectivity of the metacercariae are crucial aspects regarding the infection risk of definitive hosts in a given area (Boray and Enigk, Reference Boray and Enigk1964). The viability and infectivity of metacercariae may be analysed whether by in vitro or in vivo methods. The in vitro methods include the verification of the refractile appearance of the excretory granules as the most simple and used criterion. Checking for viability may be also achieved by removing the outer cyst wall by gently pressing with a dissecting needle under a stereomicroscope, the subsequent transfer of the excysted metacercaria to a test tube with 10 mL of modified Earle saline medium, and final activation by incubation (Dixon, Reference Dixon1964, Reference Dixon1966). The in vivo methods are better to obtain significant results but require the experimental infection of a laboratory animal model, preferably a mid-sized ruminant as sheep. The Guirra sheep strain has recently proved to be equally susceptible to both F. hepatica and F. gigantica allowing for a significant pathogenicity comparison study for the first time (Valero et al., Reference Valero, Bargues, Khoubbane, Artigas, Quesada, Berinde, Ubeira, Mezo, Hernandez, Agramunt and Mas-Coma2016). Rodents such as laboratory rats (better than mice if fluke adults are to be obtained) may also be used when dealing on pathology (Valero et al., Reference Valero, Santana, Morales, Hernandez and Mas-Coma2003, Reference Valero, Navarro, Garcia-Bodelon, Marcilla, Morales, Garcia, Hernandez and Mas-Coma2006a, Reference Valero, Girones, García-Bodelon, Periago, Chico-Calero, Khoubbane, Fresno and Mas-Coma2008) or immunology aspects (Girones et al., Reference Girones, Valero, Garcia-Bodelon, Chico-Calero, Punzon, Fresno and Mas-Coma2007), as this animal model appears more appropriate than ruminants for the extrapolation of results to humans. Hamsters for F. hepatica (Ashrafi et al., Reference Ashrafi, Valero, Massoud, Sobhani, Solaymani-Mohammadi, Conde, Khoubbane, Bargues and Mas-Coma2006a, Reference Ashrafi, Valero, Forghan-Parast, Rezaeian, Shahtaheri, Hadiani, Bargues and Mas-Coma2006b) and rabbits for F. gigantica (Prasad et al., Reference Prasad, Ghosh, Bhatnagar and Santra1999) may also be used to assess metacercarial viability and infectivity.
There have been other studies, including the description of methods and techniques for the analysis of the viability and infectivity of metacercariae for the species F. hepatica (Wikerhauser, Reference Wikerhauser1960; Tielens et al., Reference Tielens, Van Der Meer and Van Der Bergh1981; Luzon Peña et al., Reference Luzon Peña, Rojo-Vazquez and Gomez-Bautista1995). Several other studies have focused on the same aspects in F. gigantica (Hanna et al., Reference Hanna, Baalawy and Jura1975; Kimura and Shimizu, Reference Kimura and Shimizu1978; Yoshihara et al., Reference Yoshihara, Hazeyama, Kato and Goto1995; Prasad et al., Reference Prasad, Ghosh, Bhatnagar and Santra1999).
Different factors shall be considered when analysing the viability and infectivity of fasciolid metacercariae, mainly (i) definitive host isolate, (ii) time, (iii) seasonality, (iv) temperature, (v) humidity (including the counteracting dryness, desiccation and evapotranspiration) and (vi) direct sunshine. All these factors should be taken into account to understand the transmission, the epidemiology and the infection risk of human fascioliasis in an endemic area.
Experimental studies carried out within the same endemic area of the Northern Bolivian Altiplano showed that there are no differences in the infectivity of the F. hepatica metacercariae between different isolates (Valero and Mas-Coma, Reference Valero and Mas-Coma2000). Moreover, the development of the adult stage of F. hepatica proved to not depend on the isolate of origin (sheep, cattle, pig, donkeys) but on the host species which it infects (Valero et al., Reference Valero, Darce, Panova and Mas-Coma2001, Reference Valero, Panova, Comes, Fons and Mas-Coma2002). Additional studies demonstrated that flukes from secondary reservoirs as pigs and donkeys involve the same potential risk as those from the main one's sheep and cattle (Mas-Coma et al., Reference Mas-Coma, Rodriguez, Bargues, Valero, Coello and Angles1997). The absence of differences between isolates from different reservoir species implies the risk of human infection linked to the closeness of humans to livestock independently from the reservoir species, as it occurs in poor rural areas of developing countries. In the Northern Bolivian Altiplano, a hyperendemic area with the highest human prevalences and intensities known, humans and livestock overnight together inside houses for a natural heating in a very high altitude area at 3800–4100 m where nighty temperatures decrease pronouncedly (Mas-Coma et al., Reference Mas-Coma, Angles, Esteban, Bargues, Buchon, Franken and Strauss1999b). A similar behaviour occurs in the Nile Delta region of Egypt, where the presence of cows, buffaloes and/or goats in the household proved to be a high-risk factor (Curtale et al., Reference Curtale, Mas-Coma, Hassanein, Barduagni, Pezzotti and Savioli2003). Human traditions have influence in this inter-relationship, as for instance in Ethiopia where ownership of two species, sheep and/or cattle, appeared significantly associated with human fascioliasis risk (Fentie et al., Reference Fentie, Erqou, Gedefaw and Desta2013), or in Baños del Inca, Cajamarca, Peru, where a similar risk factor only concerns one reservoir species, cattle, whose raising in the neighbourhood of the home appears associated with child infection (Rodriguez et al., Reference Rodríguez, Rivera, Del Valle, Cerna, Hoban, Chilón and Ortiz2018).
Metacercarial infectivity is dependent upon storage time, being lower when metacercariae are older. Metacercariae can survive for long periods, but their viability and infective capacity decreases along time (Boray and Enigk, Reference Boray and Enigk1964; Over and Dijkstra, Reference Over and Dijkstra1975). Unfortunately, however, quantitative studies on metacercarial survival tested in vitro are difficult to compare, as the laboratory conditions were not standardized. When analysing the viability of metacercariae of F. hepatica with the Wikerhauser test, the decrease of the metacercarial viability along time was observed, so that the mortality during the first 3–4 months was low whereas the survival of metacercariae at 12 weeks decreased to only somewhat higher than 60% (Over and Dijkstra, Reference Over and Dijkstra1975). In in vivo studies, the maximum longevity was 31 and 48 weeks using doses of 20 and 150 metacercariae per rat, respectively, although in the latter case only a very low percentage was viable (Valero and Mas-Coma, Reference Valero and Mas-Coma2000).
The metacercariae of F. hepatica may survive for long periods at low temperatures if the level of moisture is sufficient, but they are susceptible to desiccation and to temperatures over 25 °C (Boray, Reference Boray1969). In contrast, high humidity associated with heavy rainfall and moderate temperatures may herald hyperendemicity in herbivorous animals. In very humid grass, they may keep their infective capacity for 8 months (Olsen, Reference Olsen1947). They are moreover pronouncedly resistant to very low temperatures. In refrigerated water, they may be viable up to 11 months (Over and Dijkstra, Reference Over and Dijkstra1975). When freezing at −2 °C their maximum survival reach 3 months, lower temperatures accelerating their death, although they resist temperatures below zero better than desiccation (Boray, Reference Boray1969).
At high temperatures, their viability decreases progressively and thus at 10 °C they are infective up to day 130, at 25 °C up to 36 days and at 30 °C only 14 days (Boray and Enigk, Reference Boray and Enigk1964). Similar results were obtained in Brazil (Müller et al., Reference Müller, Berne, Raffi, Jesus, Paulsen and Sinkoc1999).
Metacercarial cysts are killed by excessive heat and dryness (Mas-Coma and Bargues, Reference Mas-Coma and Bargues1997). Studies on excystment of F. hepatica metacercariae induced in vitro showed that the mortality of metacercariae was much higher in a moist environment than when being immersed in water (Luzon-Peña et al., Reference Luzon Peña, Rojo-Vazquez and Gomez-Bautista1995). A similar result was found in F. gigantica in the lowland Indonesian irrigated rice paddies, where metacercariae immersed in water remained viable longer than those allowed to desiccate and their viability was promoted by decreasing temperature and increasing humidity (Suhardono et al., Reference Suhardono, Roberts and Copeman2006b). A 70% humidity is considered to be sufficient for a long survival of metacercariae, above all when at low temperatures (Boray and Enigk, Reference Boray and Enigk1964). At 90% relative humidity they did not surpass 27 days at 20 °C, whereas under the same humidity but at 10 °C they kept viable up to 122 days.
The scarce resistance of metacercariae of F. hepatica when exposed to direct sunshine or desiccation, opposite to their survival capacity under high humidity conditions, has been observed several times (Olsen, Reference Olsen1947; Boray, Reference Boray1969). In F. gigantica, exposure to direct sunlight killed metacercariae within 8 h and lead the authors to suggest the option of exposing fresh rice stalks to direct sunlight before feeding them to livestock, as a way for farmers to reducing infection of their animals (Suhardono et al., Reference Suhardono, Roberts and Copeman2006a).
The influence of all these factors and their variability throughout the year in an endemic area define the seasonality of the transmission of the disease and underlies the high susceptibility of fascioliasis to climate (Ollerenshaw and Smith, Reference Ollerenshaw and Smith1969; Fuentes et al., Reference Fuentes, Valero, Bargues, Esteban, Angles and Mas-Coma1999) and to climate changes (Mas-Coma et al., Reference Mas-Coma, Valero and Bargues2009b). Veterinary studies have distinguished two main transmission models. A model of the second part of the year, with low transmission in spring and very high in autumn, with the higher incidence at the end of summer and beginning of autumn (August–September with the possibility of prolongation up to December–February) is the one followed in the majority of European countries (Irland, Denmark, The Netherlands, France, Scottland), given areas of Australia (northern Victoria) and the USA (Idaho) (Ross, Reference Ross1967, Reference Ross1977; Urquhart et al., Reference Urquhart, Doyle and Jennings1970; Over and Dijkstra, Reference Over and Dijkstra1975; Shaka and Nansen, Reference Shaka and Nansen1979; Meek and Morris, Reference Meek and Morris1979; Craig Hoover et al., Reference Craig Hoover, Lincoln, Hall and Wescoo1984; Mage, Reference Mage1989a, Reference Mage1989b). The other model, in which the first part of the year is the most risky for animal infection, is observed in Louisiana, central area of Florida and Gulf Coast of the USA and in New Zealand (Harris and Charleston, Reference Harris and Charleston1976; Craig and Bell, Reference Craig and Bell1978; Malone et al., Reference Malone, Loyacano, Hugh-Jones and Corkum1984/85; Boyce and Courtney, Reference Boyce and Courtney1990).
Regarding human endemic areas, different scenarios are found. In the Northern Bolivian Altiplano, the stability of the climatic factors throughout the year at the very high altitude allows for continuous populations of the lymnaeid vector linked (due to a high evapotranspiration rate eliminating temporal water bodies) to permanent collections of freshwater deriving from the snow of the neighbouring Andean chain and consequent human infection by metacercariae along the whole year (Mas-Coma et al., Reference Mas-Coma, Angles, Esteban, Bargues, Buchon, Franken and Strauss1999b; Fuentes et al., Reference Fuentes, Valero, Bargues, Esteban, Angles and Mas-Coma1999). In the lowland endemic region of the Nile Delta in Egypt, human infection appears to be seasonal according to the Mediterranean climatic conditions in northern Africa and following the aforementioned first model of higher human infection risk in the second part of the year (Farag et al., Reference Farag, Salem, Khalil and Farahat1993).
It should however be considered that a global change aspect as artificial irrigation may modify the timely infection risk in humans and also animals, as in areas with wide implementation of rice fields according to the local tradition of rice culture management (Valero et al., Reference Valero, Marti, Marcos, Robles and Mas-Coma1998), as for instance in the rice fields surrounding the city of Rasht on the lowlands of the Guilan province in Iran (Ashrafi et al., Reference Ashrafi, Valero, Peixoto, Artigas, Panova and Mas-Coma2015). A similar modifying impact is the one yielded by the very wide irrigation system in the arid zones of the Punjab province, Pakistan, where a natural a priori monoseasonal human infection risk related to the monsoon rainfall has artificially become biseasonal due to a winter man-made irrigation of crop fields (Afshan et al., Reference Afshan, Fortes-Lima, Artigas, Valero, Qayyum and Mas-Coma2014).
Prevention measures
Introductory considerations
The large heterogeneity of the human fascioliasis infection sources underlies a considerable control complexity linked to the many different ways the parasite may follow for successfully accessing the definitive host.
Cercariae shed by the lymnaeid snail swim for a short time (1 h) until contacting a solid support. They then lose their tails and quickly encyst, changing into metacercariae and become infective within 24 h after encystment (Mas-Coma and Bargues, Reference Mas-Coma and Bargues1997). The opportunistic ability of metacercariae to attach to solid objects is mainly marked by this short life of the swimming cercariae. The consequence is that only plants located close to where the lymnaeid is shedding the cercariae will be used for the attaching encystment. Therefore, the ecology and ethology of the lymnaeid snail vectors of both F. hepatica and F. gigantica in the endemic area in question become crucial. In that sense, the very wide spectrum of situations suitable for lymnaeid snails is to be considered, mainly the amphibious behaviour of the main lymnaeid snail vectors of both F. hepatica and F. gigantica, and the pronounced capacity of these snail vectors to adapt to water collections of different characteristics: (i) from below sea level up to the very high altitude, (ii) from temporary to permanent water bodies, (iii) from clean to eutrophic waters, (iv) from the open sylvatic field to anthropophilic and artificial habitats, (v) from overwintering by hibernation to oversummering by aestivation and (vi) from stable populations to moving/spreading populations thanks to their selfing allowing a single passively transported specimen to quickly give rise to a new population in the newly colonized habitat.
Of a very high importance is the capacity of Fasciola to give rise to metacercariae floating in the water and the long survivorship of attached and floating metacercariae in water. This means that fascioliasis is one of the few parasitic diseases which may infect humans by both food ingestion and water drinking. But additional to this, the interacting possibilities of these two ways of transmission gives rise to many different human infection sources as a result of washing, soaking and humidity keeping transport. This underlies human infections by consumption of not only very different freshwater edible vegetables by ingestion, sucking, chewing or stripped with the teeth, but also of a priori surprising terrestrial plants and fully unexpected aerial fruits or subterraneous tubercles, without forgetting the potential contamination of kitchen utensils and other objects when washing. All these, together with the low specificity of these liver flukes at mammal reservoir species level, including very numerous domestic livestock but also sylvatic herbivore and omnivorous species, conform a tremendous diffusion capacity to Fasciola species which allows understanding the worldwide distribution of this disease and consequently worldwide human infection risk. Moreover, this already speaks about the high difficulties in controlling fascioliasis everywhere.
Additionally, fascioliasis reunites the main characteristics to be a disease highly susceptible to the impacts of climate change and global change, namely (i) it is vector-borne by lymnaeid snails; (ii) shows relatively low specifity at vector level, i.e. it is able to use many different lymnaeid species distributed throughout Europe, Africa, Asia, the Americas and Oceania; (iii) it is zoonotic; (iv) it shows very low specificity at definitive host level including very numerous domestic and sylvatic animals; (v) there is no sterile immunity at definitive host level, i.e. infection does not generate a refractory effective status postinfection, e.g. after cleaning by successful treatment; and (vi) there is no premunition, i.e. running infection does not protect against parasite accumulation after reinfection. The impacts of climate change and global change have been widely observed at the veterinary level and recently proved on human fascioliasis too (Mas-Coma et al., Reference Mas-Coma, Valero and Bargues2009b; Afshan et al., Reference Afshan, Fortes-Lima, Artigas, Valero, Qayyum and Mas-Coma2014). Human fascioliasis appearance in new areas favoured transmission leading to increasing prevalences and intensities, and geographical spread of the disease, represent additional worrying scenarios and present-future challenges for control.
The heterogenic scenario
The aforementioned complex scenario becomes even more challenging from the control point of view when considering differences in diet, behaviour, traditions and habits of humans according to the different endemic areas and countries.
Differences in human infection sources and human infection risk according to the different areas and countries significantly complicate the defining of fascioliasis control measures at the global level. Both individual prevention and general control measures should adapt to the characteristics of fascioliasis transmission at the local level. Extrapolating given measures which were successful in an endemic area to another may be futile, inadvisable, difficult or even impossible to perform. An example would be, as was suggested, trying to apply the measures used in the Nile Delta region in Egypt to the Northern Altiplano of Bolivia. Factors involved in the disease transmission are too much different, e.g. respectively: (i) presence of F. hepatica, F. gigantica and hybrids vs presence of only F. hepatica; (ii) lowland (at sea level) vs very high altitude (3800–4100 m a.s.l.); (iii) origin of water (irrigation canals vs. streams and efflorescences from phreatic layers originated from Andean snow); (iv) different amphibious and aquatic lymnaeid vector species vs only one amphibious vector species; (v) presence of buffaloes and absence of pigs vs opposite situation; (vi) seasonal vs permanent transmission; (vii) low vs very high evapotranspiration; (viii) low-temperature variability within 24 h vs highly changing ones within 24 h; (ix) Muslim vs Aymara traditions and habits; (x) herd management vs few animal familial owners; (xi) Mediterranean vs high altitude Indian diet, (xii) typical villages vs open communities; or (xiii) presence vs absence of vegetable culture fields. Moreover, studies demonstrated that similar control measures were not appropriate even for endemic areas with similar characteristics, sharing the same fasciolid flukes and lymnaeid vectors, as in the cases of Egypt and Iran (Mas-Coma et al., Reference Mas-Coma, Valero and Bargues2009a).
Therefore, a made-to-measure suit is recommended for each human endemic area to assure efficacity. This is the way WHO adequately followed for the implementation of a successful pilot triclabendazole preventive chemotherapy intervention in different countries of Asia, Africa and Latin America (WHO, 2007, 2008). The appropriateness to apply different control strategies in neighbouring or close countries is sometimes not easy to explain to the respective health responsibles. The need for a minimum knowledge baseline of the local characteristics of the fascioliasis transmission to allow for the adequate design of the control measures specific for an endemic area implies previous multidisciplinary studies on the disease in the area in question. Health responsibles should take into account that individual prevention and general control measures for fascioliasis noted in textbooks and non-specialized websites may not be effective in their area or country. They should moreover consider that different endemic areas inside the same country may not necessarily be similar enough as to need identical measures. Fascioliasis is a disease in that the feeling of having lost efforts, time and funds for nothing is not rare at all.
Within this context, one should moreover consider several situations and factors when analysing whether individual patients or results of surveys carried out on affected human communities:
• Subject(s) infected in a developed country or in a developing country where there may be the absence of potable water access to dwellings through a clean water supply network, lack of sewage system for stool elimination, and absence of any control on food products and food markets.
• Inmigrants who keep their diet, traditions and habits in the reception country; infections by consumption in European countries of khat imported from African countries is a good example (Doherty et al., Reference Doherty, Price, Moody, Wright and Glynn1995; Cats et al., Reference Cats, Scholten, Meuwissen and Kuipers2000; De Bree et al., Reference De Bree, Bodelier and Verburg2013).
• Infections of travellers and migrants, up to a level that fascioliasis should today be included in the list of diseases to be enhanced in Travel Medicine. Europe is the continent where more imported cases have been reported in many countries. More cases would have been probably reported in Europe if fascioliasis would be a reportable disease. In the Americas, most of the reports concern cases diagnosed in the USA. Relative few patients have been diagnosed in studies on travellers performed in Asia. In Africa, most cases were reported in Maghreb countries (Ashrafi et al., Reference Ashrafi, Bargues, O'Neill and Mas-Coma2014);
• Travellers and physicians should consider the risk posed by the lack or insufficient knowledge about the local human diet, traditions and habits in the country or area travellers and migrants visit. Different kinds of travellers have been involved in human infection reports: business travellers, tourists, migrants, expatriated workers, military personnel, religious missionaries, and refugees (Ashrafi et al., Reference Ashrafi, Bargues, O'Neill and Mas-Coma2014).
• Despite fascioliasis being a typical disease of rural areas, it should be considered that human affection may be diagnosed in urban inhabitants who became infected whether on field trips (Mera y Sierra et al., Reference Mera y Sierra, Agramunt, Cuervo and Mas-Coma2011) or in the city itself by consumption of field-contaminated vegetables transported and afterwards acquired in uncontrolled food city markets or bazaars (Gaillet et al., Reference Gaillet, Liance, Rivollet and Houin1983; Sadykov, Reference Sadykov1988; Hughes et al., Reference Hughes, Spithill, Smith, Boutlis and Johnson2003; Kaya et al., Reference Kaya, Demirci, Demirel, Aridogan, Ozturk and Korkmaz2006) and eaten at home, in restaurants or even high standing hotels (as unreportedly once happened in Lima, Peru). The capacity of the metacercariae to keep their viability and infectivity for some time outside of water, mainly if kept under wet conditions, underlies human infections outside the field where transmission occurs. The tradition to freshen the vegetables to be sold in the market in order to keep and improve their aspect for attractive exposure allows the metacercariae to prolong their viability.
• People in developed countries should be informed about the fascioliasis infection risk inherent to the recent fashion to ‘go green’ as a healthy approach to the modern artificial lifestyle in today highly developed societies.
• Among adult patients, the higher infection risk in subjects working in professions implying occupations close to the life cycle of Fasciola should be taken into account, mainly linked to (i) livestock management (farmers, family owners), (ii) lymnaeid snail habitats (workers in plant cultures needing extensive irrigation, as in the case of rice fields, or in water ponds where fish is fed with livestock manure or human ‘night soil’) and (iii) edible vegetables suitable for metacercariae attachment, both freshwater and those terrestrial but needing extensive irrigation or washing/soaking for marketing (sellers in uncontrolled markets and bazaars; women at home producing beverages from contaminated plants and afterwards sold at non-licensed small mobile street stalls, as in most developing countries).
• Differences in livestock management according to the different local traditions depending on the endemic areas are of high importance, given that closeness to livestock usually appears as a high-risk factor; ethnic traditions relying on children and/or women for livestock field management (Fig. 10D, E), mainly small herds belonging to a family, in part underlie the higher prevalences and/or intensities in children and women in human hyperendemic areas (Mas-Coma et al., Reference Mas-Coma, Angles, Esteban, Bargues, Buchon, Franken and Strauss1999b).
• The higher infection risk in children in the human hyperendemic areas, but also the numerous reports of infection of children in animal endemic areas, rely on the fact that child behaviour typically includes the habit of putting all kind of sylvatic herbs into the mouth for eating, sucking, chewing or stripping with the teeth, and also drinking from natural water collections of different types (streams, man-made canals, ponds, etc.), along their way to school in rural areas, in field trips, when playing outside, etc. Many such histories have been documented in reports on child infections everywhere.
• A pragmatic way to facilitate the interpretation of data furnished by the anamnesis of a patient, but also to obtain a fast decrease of prevalences and intensities by education or specific forecasting methods in an endemic area surveyed, is to distinguish between the main human infection sources and other sources in the local area in question. Differences between the most frequent and the secondary sources usually appear to be very marked in an endemic area. In several areas, there is usually but only one main infection source, as watercress in Europe.
Physicians should have sufficient knowledge about not only the many different potential human infection sources, but also on the characteristics of the different phases of the disease to enable for a correct interpretation of the information provided by the patient and, after cleaning by successful treatment, hence appropriately recommend him to further avoid repeating what underlied his infection by Fasciola. The following main aspects should be considered when trying to assess the infection source and when the infection occurred:
• Symptom onset and appearance of biochemical marker alterations normally after a few days subsequent to infection (usually less than 1–2 weeks), but delayed cases of 6 weeks or 2–3 months have rarely been described (Mas-Coma et al., Reference Mas-Coma, Agramunt and Valero2014b; Valero et al., Reference Valero, Bargues, Khoubbane, Artigas, Quesada, Berinde, Ubeira, Mezo, Hernandez, Agramunt and Mas-Coma2016), without forgetting that asymptomatic infected subjects have also been reported.
• First usual symptoms include fever, asthenia and digestive manifestations (Mas-Coma et al., Reference Mas-Coma, Agramunt and Valero2014b).
• The initial migratory or acute phase lasts 3–4 months in humans.
• Biliary or chronic phase starts 3–4 days before first appearance of eggs in stools and consequently presence of eggs in stools.
• Beginning of the chronic phase is 1–2 weeks delayed in infection by F. gigantica when compared with F. hepatica (Valero et al., Reference Valero, Bargues, Khoubbane, Artigas, Quesada, Berinde, Ubeira, Mezo, Hernandez, Agramunt and Mas-Coma2016).
• Clinical differentiation by symptoms between acute and chronic phase may be misleading or overlap in reinfections (usual in children in hyperendemic areas).
• Absence of sterile immunity allowing for reinfections after successful cleaning by appropriate treatment.
• Absence of premunition allowing for reappraisal of acute symptoms during the chronic phase and subsequent accumulation of adult flukes in the liver increasing pathology due to the higher burden.
• Available coprological, serological and blood techniques and tests for human diagnosis have been recently reviewed in detail and their advantages and disadvantages in the different epidemiological situations of fascioliasis critically updated (examples of problems are infections by adults not producing eggs, delayed negativization of serological tests after successful treatment, spurious infections, uselessness of coprological egg detection during the acute phase, ectopic infections, etc.) (Mas-Coma et al., Reference Mas-Coma, Bargues and Valero2014a).
• In human hyperendemic areas, infected subjects are almost always diagnosed in the chronic phase and therefore the most useful diagnostic method for the detection of adult flukes in the bile ducts would be of great value, as is the case of cholangiography by endoscopic retrograde cholangiopancreatography (ERCP) [some technical limitations make bile duct detail obtained by ultrasound, computed tomography (CT) or magnetic resonance cholangiopancreatograpy (MRCP) imaging methods less useful than those obtained by ERCP]. Unfortunately, ERCP is neither usually available in poor rural areas nor in the neighbouring villages in low-income developing countries nowadays.
Moreover, physicians should consider the problem posed by fascioliasis because of not being considered a reportable disease. So, the frequency of human infection in an area may be pronouncedly underestimated if the physician only considers published reports or official statistics provided by the local or national health authorities.
Additionally, physicians dealing with individual patients, and health authorities responsible for the design and implementation of general control measures in an endemic area, should take into account the great difficulties in changing centenary old local traditions, to avoid frustration after the verification of the lack of success of the efforts applied. Such health problematic centenary old traditional behaviour appears in many human endemic areas of fascioliasis. The impossibility faced in the Nile Delta endemic region in Egypt when trying to implement the construction of ‘washing units’ in a village besides the one in which the washing unit strategy very successfully allowed for a drastic prevalence reduction, illustrates how difficult it may be to change traditions of the inhabitants of such rural areas. Local women rejected the proposal of using such new installations and decided to go on following their familiar traditions of washing in the canals.
Adequate specific training of physicians and other sanitary personnel working in hospitals and outpatient attendance centres in the rural environment and neighbouring villages and cities is recommended for human fascioliasis endemic areas.
Physicochemical agents against metacercariae
Studies on the viability and infectivity of Fasciola metacercariae performed on the field to assess their repercussions on the transmission and epidemiology of the disease in livestock have been numerous. Contrarily, only a very few experimental studies have been made on the resistance of the metacercariae to analyse the effects of different physicochemical agents useful for preventive purposes in the market or the kitchen to avoid human infection at home or animal infection in a controlled stall. Worth noting here is that prevention of livestock infection can be achieved if only the portion of fresh rice stalks or other plants growing above the water level is fed to the animals (Mahato and Harrison, Reference Mahato and Harrison2005). Exposing fresh rice stalks to direct sunlight before feeding them to livestock is another option suggested for the same purpose (Suhardono et al., Reference Suhardono, Roberts and Copeman2006a).
Moreover, these few studies have not followed a standardized method, as for instance regarding a crucial initial factor as the age of the metacercariae used, so that significant comparisons cannot be made. The complexity of the obtaining of metacercariae under standardized conditions in the laboratory and the need for experimental infection of a laboratory animal model as the only complete method for the verification of the viability and infectivity of Fasciola metacercariae undoubtedly underlie the reason for the very low number of such studies performed so far.
Results obtained in these few experimental studies are summarized in Table 1. Unfortunately, only a few physicochemical agents have been assayed and moreover under an only short variation of doses and time. Temperature appears as the only exception. Fortunately, anyway, both Fasciola species have been used for such assays.
Table 1. Effect of physicochemical agents on the viability and infectivity of metacercariae of Fasciola hepatica and F. gigantica
T, temperature.
The following main conclusions may be reached about the resistance of the metacercariae of Fasciola from both the experimental and the field results:
• they may reach a very long survival, although their viability decreases with their increasing age;
• they are highly resistant to chemical products;
• given chemicals may detach metacercariae from the vegetables but do not kill them and consequently, subsequent action for their elimination is still needed;
• several experimental results with chemical products appear to be in need of verification, according to the results obtained in the repeated assays with potassium permanganate;
• the physical methods may be more effective, as shown by the temperature;
• temperature effects indicate that cooking/heating vegetables and boiling water may be effective if the temperature reached and the applied time is sufficient;
• contrarily, refrigerating and freezing do not appear to be recommendable methods due to the high resistance of the metacercariae to the low temperatures, even those lower than 0 °C;
• filtration of water seems to be an affordable method, as shown by the success obtained by the ‘washing units’ furnishing water cleaning by a filtration system of the sand type filtration usually applied to swimming pools;
• the size of the metacercariae, with a diameter of outer cyst wall of 180–206 µ m in F. hepatica, and 180–330 µ m and occasionally up to 650 µ m in F. gigantica (the large diameter variability in the latter species in Asia suggests including hybrid forms) easily allows for the production of the appropriate size pass filters which could be implemented in the water faucets or better inside the entrance tube providing water for the whole dwelling;
• sunshine (and artificial ultraviolet radiation) and drying (and artificial dryers) are additional potentially effective agents which are in need of further research.
Thus far, heat appears to be the only sure way to kill the metacercariae. Indeed, the importance of boiling drinking water on diarrhea and pathogen-specific infections in low- and middle-income countries has recently already been emphasized in general (Cohen and Colford, Reference Cohen and Colford2017). Anyway, temperatures applied should be assured to be sufficient, because assays made with lower ones proved to be ineffective in Fasciola. In China, human infection rarity has been linked to the tradition of cooking the edible vegetables (Chen and Mott, Reference Chen and Mott1990). Unfortunately, in other countries there is usually no tradition to heat up or boil the freshwater vegetables involved in fascioliasis transmission, nor are these vegetables of a convenient contexture for such a measure. Moreover, in several human endemic areas of rural areas of developing countries, there is usually neither electricity nor gas network available in the dwellings as to allow for an easy way to heat up or boil the food.
Potassium permanganate has been suggested to be the most effective preventive chemical tool for killing metacercariae attached to leaves and vegetables used in salads (El Sayed et al., Reference El Sayed, Allam and Osman1997; El-Zawawy et al., Reference El-Zawawy, Ali and Allam2003; Hassan et al., Reference Hassan, Shoukary, El-Motayam and Morsy2008). However, 5-min tests of potassium permanganate effects showed that metacercarial viability was not affected even at the very high doses of 300, 600 and 1200 mg L−1 (Ashrafi et al., Reference Ashrafi, Valero, Massoud, Sobhani, Solaymani-Mohammadi, Conde, Khoubbane, Bargues and Mas-Coma2006a; Reference Ashrafi, Valero, Forghan-Parast, Rezaeian, Shahtaheri, Hadiani, Bargues and Mas-Coma2006b).
It is evident that more research on the potential use of physical and chemical agents for the killing of metacercariae for preventive purposes in-house kitchen and also markets is needed. In front of the present insufficiency of these methods, people, physicians, other sanitary personnel and health authorities should be aware of other individual prevention measures to avoid infection and general measures for fascioliasis control.
Individual measures to avoid infection
People living in or visiting fascioliasis rural disease transmission areas should consider that human infection risk pronouncedly differs between human endemic and animal endemic areas (Mas-Coma et al., Reference Mas-Coma, Esteban and Bargues1999a). In human hyperendemic areas, infection risk is highest in children because of their behaviour including habits of putting all kind of plants in their mouths for eating, sucking, chewing or stripping with own teeth, as well as drinking from natural water collections and unsafe fountains. In these rural areas, the second level of infection risk is for women, mainly due to their activities linked to food preparation in the kitchen but in given areas also to the tradition of women to be in charge for livestock management.
In areas with the seasonal transmission of the disease, year periods with higher incidence risk should be considered, such as rainy and post-rainy periods or watercress season. However, the considerable resistance to physicochemical agents and the long-term viability and infectivity of fasciolid metacercariae explain why sometimes people become infected in a year period not included in the typical disease seasonality when most of the human cases are detected.
The higher infection risk for people living or working close to livestock should be taken into account as a significantly high-risk factor. Sharing of contaminated food and/or water underlie frequent outbreaks involving a short number of infected persons whether belonging to the same family or activity group.
Inhabitants and visitors of an endemic area should consider that the usual diet and traditions of the area in question and that plant species involved in the transmission of fascioliasis differ according to geographical zones and human dietary habits. So, risky vegetables may be very different in the area visited than those well known in the original area of the visitor.
People should be aware of the fact that plant species involved in fascioliasis transmission may not necessarily be the same ‘at table’ (through vegetables making part of the normal diet) than in the field. Thus, ingestion, sucking, chewing or stripping with own teeth of vegetables directly taken from nature, and which may not necessarily make part of the usual local human diet, should be taken into account and let know mainly to children.
Special care should be focused on watercress as the plant most involved worldwide, mainly wild but also the controlled cultivated watercress. It is the main source and almost only plants referred to in many developed countries, such as in Europe. Individual infections (Hughes et al., Reference Hughes, Spithill, Smith, Boutlis and Johnson2003; Kaya et al., Reference Kaya, Demirci, Demirel, Aridogan, Ozturk and Korkmaz2006) and even outbreaks (Mailles et al., Reference Mailles, Vaillant, Schepens, Aajana, Capek, Ilef, Fillebeen, Lefort, Therouanne, Volant, Flavigny and De Valk2003, Reference Mailles, Capek, Ajana, Schepens, Ilef and Vaillant2006) occurred due to the ingestion of contaminated, supposedly safe, watercress acquired in city markets put a question mark of up to which level total safety may be reached in controlled watercress cultures.
This does not mean, however, that other plant species secondarily involved, as dandelion, can be neglected. In other regions, the human infection risk may be more linked to other plant species and care should be focused on them. The consequence of the importance to know, which plants are risky in which endemic area, is easily deduced. Similarly, the danger inherent to local food specialities made from sylvatic plants should not be overlooked, nor the one linked to sylvatic plants sold in city markets and which differ markedly according to local dietary habits.
The mistake of considering that only leaves of freshwater vegetables are risky should not be made. On one side, seeds and edible stems may also transmit if contaminated when washed, soaked, or freshen for marketing, and in the case of stems when the plant is one in which it is immersed in water. On the other side, not only sylvatic but also cultivated aquatic and terrestrial plants have been seen to be involved in different areas of Asia and Africa, respectively. Washing, soaking or freshening for marketing, are procedures at the origin of the plant contamination when using contaminated water.
The risk inherent to vegetables imported into and consumed in developed countries, but produced in and transported from developing countries under sufficient humidity conditions, has recently proved to be an additional way leading to human infection. The infection reports of subjects infected by khat consumption in Europe after non-controlled importation is a good example (Doherty et al., Reference Doherty, Price, Moody, Wright and Glynn1995; Cats et al., Reference Cats, Scholten, Meuwissen and Kuipers2000; De Bree et al., Reference De Bree, Bodelier and Verburg2013).
Workers in field cultures of crops needing important irrigation and farmers and owners of livestock have to know about the risk of allowing domestic ruminants to move around and even inside the field cultures (Fig. 10F) and avoid such situations by implementing appropriate fences to prevent livestock to enter in the fields. For the same reason, neither human ‘night soil’ nor livestock manure should be used to fertilize the cultivated fields. In Asia, similar practices for fertilizing water ponds in which fish are grown should also be avoided. In the same sense, care should be taken when cleaning latrines, avoiding to spread fecal samples into water collections inhabited by lymnaeids and frequented by livestock.
In endemic lowland areas where plant cultures are viable, sometimes the irrigation canals and irrigated fields are located just beside the dwellings and even schools of peripheral city suburbs, thus becoming high infection risk places for the children.
Private gardens used for the familiar production of vegetables are at risk if measures are not taken to avoid lymnaeid snail colonization and contamination by eggs shed by livestock moving in the garden neigbourhood. Moreover, using fertilizers other than human ‘night soil’ or livestock manure should be assured.
People use to think that plant species which grow in soil flooded with water are more juicy and tasty. It must be remembered that such flooded areas constitute ideal habitats for the amphibious lymnaeid snail vectors which may be shedding cercariae if the place is frequented by livestock, although animals may be absent in the moment of plant collection. Thus, collecting aquatic, semiaquatic or even terrestrial plants in such flooded areas, even if the area is not flooded in the moment of plant collection, for their subsequent eating, sucking, chewing, or stripping with teeth should always be avoided.
Similarly, popular local dishes, appetizers and condiments made from sylvatic aromatic plants may represent a high risk for individual infections, outbreaks when shared by family members or groups, and even for disease spread when given as presents to people from other areas where such local foods are not produced nor available, as in Iran (Ahsrafi et al., Reference Ashrafi, Valero, Massoud, Sobhani, Solaymani-Mohammadi, Conde, Khoubbane, Bargues and Mas-Coma2006a, Reference Ashrafi, Valero, Forghan-Parast, Rezaeian, Shahtaheri, Hadiani, Bargues and Mas-Coma2006b). The production of local beverages and juices made from local plants, e.g. emollients and above all alfalfa juice, pose a similar problem in Peru (Raymundo et al., Reference Raymundo, Maco Flores, Terashima, Samalvides, Miranda, Tantalean, Espinoza and Gotuzzo2004; Marcos et al., Reference Marcos, Maco, Samalvides, Terashima, Espinoza and Gotuzzo2006) and Mexico (Zumaquero-Rios et al., Reference Zumaquero-Rios, Sarracent-Perez, Rojas-Garcia, Rojas-Rivero, Martinez-Tovilla, Valero and Mas-Coma2013), countries where drinking of these local products should be avoided.
A worrying scenario is the one linked to the wide drive to ‘natural feeding habits’ by consumption of foods non-treated by chemicals (insecticides, molluscicides) or genetically modified (transgenic plants) in the way for a supposed more healthy modern lifestyle. This fashion is leading to a marked increase in the consumption of fresh, raw/green fruit and vegetables (Broglia and Kapel, Reference Broglia and Kapel2011; Hotez et al., Reference Hotez, Alvado, Basáñez, Bolliger, Bourne, Boussinesq, Brooker, Brown, Buckle, Budke, Carabin, Coffeng, Fevre, Fürst, Halasa, Jasrasaria, Johns, Keiser, King, Lozano, Murdoch, O'Hanlon, Pion, Pullan, Ramalah, Roberts, Shepard, Smith, Stolk, Undurraga, Utzinger, Wqng, Murray and Naghavi2014).
Drinking natural water in the field in endemic areas is highly risky, whether from natural streams, artificial canals, ponds, lagoons, etc., mainly from those frequented by livestock. Useful risk markers as lymnaeid snails may be undetectable in a water collection (e.g., in between generations according to the local population dynamics, during hibernation or aestivation periods) and livestock be absent around it, but metacercariae are present and still viable in the moment of water drinking. Therefore, collecting natural water and transporting it to the dwelling for washing, drinking or food preparation at home, is a risky habitat.
Drinking water from field fountains may be risky mainly when artificial fountains are constructed in the way to be shared by humans and livestock, as in Corsica Island. Man-made fountains should quickly be repaired when broken and originating overflowing waters from which water is taken home in poor rural areas in which no other close water source is available (Fig. 7C).
Large water bodies may also represent an infection risk for humans. Therefore, swimming in recreational waters frequented by livestock should be avoided, as in Argentina (Mera y Sierra et al., Reference Mera y Sierra, Agramunt, Cuervo and Mas-Coma2011). Entering into water canals to wash animals, foods and clothes, drinking water from these waters, and pipping water from such outside waters up to dwellings, as in Egypt (Curtale et al., Reference Curtale, Mas-Coma, Hassanein, Barduagni, Pezzotti and Savioli2003), should be avoided.
Care should be taken to avoid the use of contaminated natural water from the field in endemic areas for the washing of vegetables (both freshwater and terrestrial), fruits (aerial fruits if fallen down into water), tubercles (radish, carrots), kitchen utensils and other objects. Because of that risk, measures should be taken to avoid the use of natural water to soak or freshen vegetables for preparing salads or making soups at home, as well as for subsequent marketing purposes.
Finally, the risk of consuming raw liver of livestock should not be neglected. At any rate, acquiring fascioliasis may not be very likely because of the very low probability to ingest a liver just in the moment of harbouring live immature flukes aged a very few days only. Neither should be neglected the possibility of acquiring halzoun when consuming raw liver of sheep and goats at festivals in northern African, eastern Mediterranean and Near East countries, although the capacity of Fasciola to give rise to a pharyngeal fascioliasis syndrome is still in need for definitive confirmation. Cooking of liver meet would easily solve such risk, although centenary old traditions in religious celebrations may not allow for such a solution.
General preventive measures in endemic areas
In the past, human infection was always related to animal endemics, so that prevention and control measures recommended were traditionally the same to be applied for veterinary fascioliasis, at the levels of domestic animals, snails and field (Roberts and Suhardono, Reference Roberts and Suhardono1996; Torgerson and Claxton, Reference Torgerson, Claxton and Dalton1999; Spithill et al., Reference Spithill, Smooker, Copeman and Dalton1999). However, studies on human endemic areas performed in the last two decades have shown that traditional epidemiological patterns of animal fascioliasis may not always explain the characteristics of human infection in a given area. Therefore, control measures for human fascioliasis should consider the results of the eco-epidemiological studies previously undertaken in the area concerned (Mas-Coma et al., Reference Mas-Coma, Valero and Bargues2009a).
The many different Fasciola transmission strategies and human infection sources do not make it easy for health authorities to design effective control programs. The aim of eradicating the disease in an endemic area does not seem realistic nowadays. Future ‘One Health’ or ‘Eco-Health’ initiatives still appear to be too much difficult to be implemented due to the wide simultaneous multidisciplinarity interventions needed and the numerous factors involved. Such attempts have still never been implemented and their effectiveness is therefore still pending verification.
In front of such a complexity, general preventive measures are focused against given factors and specific purposes. A good example is the worldwide WHO interventions with yearly preventive chemotherapy campaigns by means of monodose treatments of triclabendazole for human use according to made-to-measure strategies for each endemic country. The specific purpose of these interventions is decreasing pathogenicity, mainly in children, by decreasing prevalences and burdens.
Legislation may be useful in several axes, although laws appropriate for the control of fascioliasis have been implemented in only a very few countries.
An aspect which offers serious problems for the analysis of the epidemiological situations of this disease in the different countries is the consideration of fascioliasis as a non-reportable disease. So, many human infection cases remain unreported and two consequences become highly problematic, namely the underestimation of the real situation and the insufficient information about risky areas and sources for human infection in the countries. Peru has been the first endemic country to make a decisive step forward in this aspect. Vietnam is following and efforts, in the same way, are being implemented in the La Paz province in Bolivia.
Another aspect of high importance in fascioliasis transmission to humans for which legislation has been implemented is watercress. Examples of countries where a high risk by watercress consumption has been proved and consequent appropriate legislation has been implemented are France and Australia. Commercial growing of watercress should be carried out under completely controlled conditions, without access for snails and ruminants. In countries where such tradition exists, there is a detailed governmental regulation of watercress production (Fennell, Reference Fennell2006). Interestingly, the legislation for the industrial watercress cultures in France was in need for an update by including a modification to take appropriate additional measures against the potential contamination of cultivated watercress beds by small mammal hosts such as the nutria, which is able to enter through the usual fences implemented to assure isolation from livestock.
There are other aspects for which legislation is needed and may be useful. Appropriate legislation should be implemented and producers advertised about risks of using sylvatic plants for the production of popular local dishes, appetizers, condiments, beverages and juices in countries where a link between fascioliasis infection and such traditional foods and drinks have been proved.
Laws are also needed to assure the safety and control of foods to be sold in city markets and the implementation of regular inspections for the appropriate control of such public markets as well as of mobile street stalls selling foods and beverages in developing countries.
Specific legislation needs to be implemented for the control of vegetables particularly suitable for contamination by Fasciola, produced in endemic developing countries, and inadvertently transported and introduced into European or other countries by uncontrolled immigrants, such as in the case of khat.
Laws are also convenient to define safety measures for public fountain construction, mainly to avoid fountains to be shared for potable water drinking by humans and livestock. Such laws should also emphasize the need for quick reparation of the fountains when broken, water leakage and origin of overflooding in risk for lymnaeid snail colonization and livestock attraction.
Authorities should prioritize the implementation in rural areas of sewage systems for the elimination of excreta, urine and other residue, and potable water supply networks including access to dwellings when annual governmental country budgets allow to cover such costs. Absence of such networks and systems typically appears in human fascioliasis hyperendemic areas of developing countries. In front of budget difficulties for such purposes, two alternative transitory solutions may be considered: (i) the construction of latrines provided that appropriate instructions for their regular cleaning and excreta elimination will be sufficiently difused and their application controlled, and (ii) the construction of ‘washing units’ furnishing water filtered from water pipped from artificial canals or rivers; the construction and utilization of the so-called ‘washing units’, in which the water was appropriately filtered, gave rise to a marked decrease of human infection in an Egyptian locality where a high prevalence in humans was initially found (Mas-Coma, Reference Mas-Coma, Cotruvo, Dufour, Rees, Bartram, Carr, Cliver, Craun, Fayer and Gannon2004).
The implementation of electricity and gas networks including access up to individual dwellings are subsequent steps which would allow for cooking vegetables and boiling water, as well as for house heating avoiding the need for animal heating and the consequent close contact with livestock still happening today in poor rural areas.
Education is a priority axis within general control measures against human fascioliasis, but also animal fascioliasis. Special formation about the disease, its pathogenicity and symptoms, local transmission characteristics, and infection sources should be given to school teachers, children, mothers, housewives and community chiefs. Well designed, easily understandable posters sufficiently illustrated with drawings or photographs are very helpful for both school teachers and general diffusion. A minimum knowledge about the main fascioliasis characteristics in the local area should previously be obtained by appropriate research studies, to avoid teaching transmission characteristics and infection sources taken from books or other countries but which may not correspond to the local rural area in question. Such mistakes are often observed in developing countries.
Education efforts in human endemic areas should be directed to children, as the age group of higher infection risk. Specific formation of children must include aspects traditionally considered secondary and often overlooked, as for instance the need to avoid sylvatic plant-eating, sucking, chewing or stripping with the teeth, not only freshwater but also semiaquatic or terrestrial ones growing beside water collections or in flooded areas, and fruits fallen to humid soil or freshwater and tubercles as radish and carrots and fruits soaked in natural water in the field, as well as drinking water from field water collections.
Such child training should not only be made with children inhabiting the rural areas, but appears also convenient for children attending city schools but may more or less usually visit close rural areas because of family relationships, weekend trips, or summer season holidays.
Teaching lessons for the inhabitants of these endemic areas, mainly housewives, should include not only the infection risk posed by freshwater vegetables and terrestrial vegetables washed or soaked with contaminated water from the field and also those acquired in an uncontrolled city market, but also the risk of natural water drinking, bathing in freshwater bodies inhabited by lymnaeids, the use of natural water from the external milieu for kitchen purposes or food preparation, and washing whether of foods or kitchen utensils,
In an interesting study in Egypt (Fawzi et al., Reference Fawzi, El-Sahn, Ibrahim and Shehata2004), knowledge and practices of housewives concerning Fasciola and its source of transmission and methods of washing leafy vegetables were obtained through house-to-house interviews with 303 housewives. The study revealed that most housewives in all age groups and all educational levels had a poor knowledge regarding the sources of Fasciola transmission. This indicates that they do not perceive that it is transmitted through vegetables and most of them do not take appropriate measures to protect themselves and their families from this parasite. This ignorance increases the risk of infection. Knowledge that consumption of contaminated leafy vegetables was a source of Fasciola infection was indirectly proportional with age, better in younger housewives and those of secondary or higher education. A 57.7% of housewives washed leafy vegetables under running tap water and 32.7% soaked them in tap water. Only 9.6% soaked them in water mixed with a substance as vinegar, lemon juice or common salt. Previous studies showed that washing vegetables with only tap water were associated with higher Fasciola prevalence. Only 5% of those who were infected with vegetable transmitted parasites washed vegetables by soaking in water with an added substance compared with 19.6% of parasite-free housewives. Most of those adding a substance to soaking water (89.7%) used vinegar. There was no statistically significant difference regarding soakíng period between those who added a substance or those who did not. It was also considered that, other than the vegetable washing methods, inhabitants may eat outside homes and even on their way home from surrounding fields without adequately washing vegetables. This study revealed that a serious and consistent effort through public health activities is needed to educate housewives about vegetable-transmitted parasites, their transmission and ways of prevention (Fawzi et al., Reference Fawzi, El-Sahn, Ibrahim and Shehata2004).
Authorities should also play a crucial role in giving instructions on how to implement appropriate diffusion on the characteristics of the disease through different media. The radio appears to be highly convenient for diffusion in rural areas of developing countries. Besides giving instructions on which hospitals and health centres to visit for diagnosis and treatment in front of suspicious symptoms, diffusion should also deal on the risk posed by the different infection sources and other related factors, as e.g. mediatic campaigns of reminders about the beginning of the risky season.
Diffusion through the different media should sometimes also be pushed by health authorities in developed countries. The infection risks posed by the modern trend for ‘natural feeding habits’ by prioritizing the consumption of non-treated, fresh, raw/green fruits and vegetables is a good example of still insufficient appropriate information in developed countries which may have consequences for human fascioliasis.
Concluding remarks
The present analysis of the infection sources is the first time that such a deep approach considering the large heterogeneity of epidemiological situations and transmission patterns of human fascioliasis is made for all human fascioliasis scenarios known worldwide. Five main conclusions may be emphasized from a general perspective:
• Human fascioliasis infection sources include foods, water and combination of both;
• The many different human infection sources draw a complex picture which speaks about the large capacity of Fasciola to take advantage of different transmission ways and shows a prevention field pronouncedly more complicated than the one considered time ago;
• The heterogeneity of the human infection sources appear to be the result of the high capacities of the liver flukes to colonize and adapt to new, markedly different natural environments and corresponding adaptative behavioural, dietary and social characteristics of the respective human communities, thanks to the following main features:
◦ adult stage hermaphroditic and able to reproduce by both selfing and crossing;
◦ vector-borne transmission by worldwide distributed freshwater snails (suitable lymnaeids are almost everywhere) including numerous species with selfing capacity, very high multiplication capacity, different ecology, different ethology, and great colonization capacities;
◦ zoonotic, with low specificity allowing for the use of many herbivore and omnivorous domestic livestock species and also sylvatic mammals species as reservoirs;
◦ infective stage constituted by encysted metacercariae with high resistance, long survival, capacity to attach to different objects (in nature, mainly vegetables growing in lymnaeid-inhabited waters) or to float in water, and therefore to contaminate many foods and drinks;
◦ capacity to avoid giving rise to effective refractory sterile immunity and premunition at definitive host level, including humans;
• The high diversity of infection sources and their heterogeneity in different countries underlie in great part the large epidemiological heterogeneity of this human disease worldwide;
• The large heterogeneity of human infection sources allows explaining, in part, the lack of parallelism between human and animal infection rates in many areas.
Finally, a plea for action should be made regarding the need of additional research on physicochemical tools which could be useful for human prevention against this disease.
Acknowledgements
Studies of this article have been performed within the framework of the Worldwide Initiative of WHO against Human Fascioliasis (WHO Headquarters, Geneva, Switzerland). The support by the Department of Control of Neglected Tropical Diseases, World Health Organization, WHO/OMS, Geneva, Switzerland; by the Animal Production and Health Section, Department of Nuclear Sciences and Applications, International Atomic Energy Agency, IAEA Headquarters, Vienna, Austria; and by the Animal Production and Health Division, Food and Agriculture Organization of the United Nations, FAO/UN Headquarters, Rome, Italy is greatly acknowledged for their help in facilitating the international collaboration necessary for the research activities developed. At the national level, authors want to thank the collaboration by very numerous specialists regarding different aspects of management, field activities and laboratory work in the different countries. Analytical review performed within the frames of the European Network for Foodborne Parasites in Europe EURO-FBP, COST Action No. FA1408, EC, Brussels, and the Global Water Pathogen Project GWPP, UNESCO Paris and Michigan State University, USA (www.waterpathogens.org).
Financial support
Financial support obtained by Project No. SAF2010-20805 of the Ministry of Economy and Competitiveness (MINECO), Madrid, Spain; by Health Research Project No. PI16/00520, Subprograma Estatal de Generación de Conocimiento de la Acción Estratégica en Salud (AES), Plan Estatal de Investigación Científica y Técnica y de Innovación, ISCIII-MINECO, Madrid, Spain; by Project No. 2017/ACDE/001583 de Innovación para el Desarrollo of the Agencia Española de Cooperación Internacional para el Desarrollo (AECID), Ministry of Foreign Affairs and Cooperation, Madrid, Spain; by the Red de Investigación de Centros de Enfermedades Tropicales – RICET (Project No. RD16/0027/0023 of the PN de I+D+I, ISCIII-Subdirección General de Redes y Centros de Investigación Cooperativa RETICS), Ministry of Health and Consumption, Madrid; by Project No. 2016/099 of the PROMETEO Program, Programa of Ayudas para Grupos de Investigación de Excelencia, Generalitat Valenciana, Valencia, Spain; and by Project entitled ‘Fascioliasis humana en el Altiplano de Bolivia: Control de la reinfección alimentaria’ of the V Convocatoria de Proyectos de Cooperación al Desarrollo de la Universidad de Valencia de 2016, Valencia, Spain.
Conflicts of interest
None.
Ethical standards
Not applicable.