Introduction
Soil-transmitted helminthiasis (STH) is one of the commonest neglected tropical diseases (NTDs) worldwide and remains a public health problem in poor communities with enormous consequences for development (Tchuem Tchuenté et al. Reference Tchuem Tchuenté, Noumedem, Ngassam, Kenfack, Gipwe, Dankoni, Tarini and Zhang2013). A recent analysis of NTDs in SSA (sub-Saharan Africa) identified Nigeria as a country with the greatest number of cases of STH infections (Hotez and Kamath, Reference Hotez and Kamath2009) and ranked fourth or fifth globally behind China, India and Indonesia (DeSilva et al. Reference DeSilva, Brooker, Hotez, Montresor, Engels and Savioli2003; Hotez and Ehrenberg, Reference Hotez and Ehrenberg2010; Lobo et al. Reference Lobo, Velayudhan, Chatterjee, Kohli and Hotez2011; Tchuem Tchuenté et al. Reference Tchuem Tchuenté, Noumedem, Ngassam, Kenfack, Gipwe, Dankoni, Tarini and Zhang2013).
Ascaris lumbricoides (roundworm), Trichuris trichiura (whipworm), Necator americanus and Ancylostoma duodenale (Hookworms) are the commonest intestinal nematodes causing STH infection (WHO, 2012). It has been estimated that one-half of 181 million school-aged children (SAC) is infected with one or some combination of these intestinal nematodes (Brooker et al. Reference Brooker, Clements and Bundy2006). Recently, it was predicted that about 5·7 million of the 41·5 million SAC in Nigeria are infected with any species of the STH, with an overall predicted prevalence of 13·8% (Oluwole et al. Reference Oluwole, Ekpo, Karagiannis-Voules, Abe, Olamiju, Isiyaku, Okoronkwo, Saka, Nebe, Braide, Mafiana, Utzinger and Vounatsou2015). Infections have been shown to contribute to deleterious health such as anaemia, growth stunting, protein-calorie malnutrition, fatigue and poor cognitive development (Hotez et al. Reference Hotez, Brindley, Bethony, King, Pearce and Jacobson2008). The World Health Organization therefore recommended annual or bi-annual deworming of school children for STH with prevalence cut-offs of 20 or 50% in endemic school populations, respectively (WHO, 2002). However, deworming has not been able to prevent re-infection, especially in heavily contaminated endemic areas (Jia et al. Reference Jia, Melville, Utzinger, King and Zhou2012). Therefore, the integration of STH control with the provision of water, sanitation and hygiene education interventions (WASH) is thus been promoted as a complementary strategy for the elimination of STH (Freeman et al. Reference Freeman, Ogden, Jacobson, Abbott, Addiss, Amnie, Cairncross, Callejas, Colford, Emerson, Fenwick, Fishman, Gallo, Grimes, Karapetyan, Keene, Lammie, MacArthur, Lochery, Petach, Platt, Prabasi, Rosenboom, Roy, Saywell, Schechtman, Tantri, Velleman and Utzinger2013).
WASH resource programming includes access to safe water, improved sanitation and good hygiene practices and education (Freeman et al. Reference Freeman, Ogden, Jacobson, Abbott, Addiss, Amnie, Cairncross, Callejas, Colford, Emerson, Fenwick, Fishman, Gallo, Grimes, Karapetyan, Keene, Lammie, MacArthur, Lochery, Petach, Platt, Prabasi, Rosenboom, Roy, Saywell, Schechtman, Tantri, Velleman and Utzinger2013). Implementation of WASH continues to gain momentum with increased commitment from governmental and non-governmental donors through the provision of funds and resources. UNICEF plays a leading role in providing access to potable water, improved sanitation and hygiene education in rural communities and schools in Nigeria. This project commenced about a decade ago in Ogun State, due to lack of potable water and poor sanitation. However, the coverage is not universal (UNICEF, 2008; Mogaji et al. Reference Mogaji, Adeaga, Yusuff, Johnson and Ekpo2016). The maintenance of these resources is also left in the hands of the community members or schools where it is situated. Till recent, reasonable impact assessment of WASH intervention on schooling children is yet to be undertaken in Nigeria. This study therefore assesses the school-based WASH programme and burden of STH infections among schooling children in southwest Nigeria.
Materials and methods
Study area and selection of schools
This survey was undertaken in eight public primary schools in Odeda area, near Abeokuta (the capital city) of Ogun State. Odeda is one of 20 administrative units in the State, and is the pilot unit for water, sanitation and hygiene resource programming. There were 106 public primary schools in the area in 2013. Study schools were selected using a stratified random sampling procedure. Schools were first categorized into two groups; those benefitting from WASH resource programming (n = 3) and those without (n = 103) (Mogaji et al. Reference Mogaji, Adeaga, Yusuff, Johnson and Ekpo2016). The three WASH resource programming school were purposively selected. The non-WASH resource programming schools were further stratified into five clusters based on proximity. Finally, random sampling was employed in the selection of a school per cluster. Eight primary schools were selected in total for the study (Table 1). Schools with WASH intervention had support from non-governmental organizations (i.e. UNICEF) in the provision of water pumps, toilet and urinal facilities and in some cases hand-washing facilities as compared with non-WASH resource programming schools. Since intervention was not randomized, this study was purely observational. The eight schools in the study were treated individually and not as clusters since they were not close to each other (Fig. 1). However, it is expected that children in the WASH resource programming schools would collectively depend on the provided WASH resource.
Fig. 1. Map of study schools.
Table 1. List and characteristic of study schools

Selection of study participants
A total of 60 pupils per school were targeted for recruitment into the study, with a minimum number of 50 based on WHO guideline on survey for helminthiasis in schools (WHO, 2002). Selection of children in each school was carried out after stratification by their class grade from Primary 1 to 6. A quota was then allocated for each class grade with proportional allocation according to the number of students in each grade. Finally, the participating children were randomly selected. In schools where pupils were not up to 50 students, the entire pupils were encouraged to participate in the study. However, only 428 pupils consented to the study procedures and were recruited, hence giving roughly an unequal number of pupils selected across the schools.
Ethics statement
Ethical approval was granted by the ethical review board of Department of Public Health and Disease control, Ogun state Ministry of health. Permit to use the selected schools for sample collections were obtained from Ogun state Ministry of Education, Science and Technology and Ogun state Rural Water Supply and Sanitation Agency (RUWATSAN). Consent was sought and obtained from parents and guardians of the pupils after they were duly informed of the research. However, pupils oral consent was obtained verbally and documented on a child assent form. Participants were provided with single dose 400 mg albendazole treatment after the study.
Data collection
Three different survey field forms were used during the survey procedures for data collection. The pupil's form, the KAP form and the school form. The pupil's form was used to obtain each child's demographic information (name, date of birth, age and sex of the child). A questionnaire was administered on the children on Knowledge, Attitude and Practices (KAP) to obtain information on helminthiasis, sanitation, personal and environmental hygiene. A school form was used to assess the status and conditions of water, sanitation and hygiene. These include the type of water supply, number of water source, condition and type of latrines, number of latrines, latrines per population ratio, gender-segregated toilets, availability of soap for hand washing, and the presence of garbage piles around the school premises.
Determination of STH burden
A single stool sample was collected from each pupil, processed within 2 h after collection using sodium acetate-acetic acid formalin concentration method (SAF-ether) and examined for intestinal ova of STH. Two slides were prepared from 1 g of each stool sample collected for microscopy. Helminth eggs were counted for each species of STH, and the mean number of egg per gram (EPG) of stool was recorded for the examined person, from which the infection intensity estimate for the school was computed.
Scoring of status and condition of WASH resources
The type, conditions, adequacy and usage of water, sanitation and hygiene resource in both the WASH resource programming schools and non-WASH resource programming schools were assessed using a WHO/UNICEF recommended checklist for improved WASH interventions in schools (Adams et al. Reference Adams, Bartram, Chartier and Sims2009). The status and condition of the WASH resources were carefully observed during field visitations and those that met the WHO/UNICEF set standards (i.e. improved conditions) were scored one point, while those that did not meet the set standards were scored zero or a negative point as appropriate. For each WASH resource component (i.e. water component, sanitation component and hygiene component), a cumulative test score was computed and used for comparison using chi-square (χ 2) statistics (Table 2).
Table 2. Scoring template for assessing WASH status and conditions

0, Bad/Non-improved/Inadequate condition of WASH resource; 1, Good/Improved/Adequate condition of WASH resource; NA, Not applicable.
Data analysis
Descriptive statistics were used to characterize the study population. The number of EPG of stool was transformed using log (n + 1) of raw count. The differences in prevalence and intensity of STH infections between WASH and non-WASH resource programming schools were determined using χ 2 statistics, t-test and analysis of variance, respectively. Significance was set at P ⩽ 0·05.
Results
Demographic characteristics of the study schools
A total of 428 children aged 5–15 years were sampled in eight public primary schools that participated; 175 (40·9%) were from WASH resource programming schools and 253 (59·1%) were from schools without WASH support. A total of 226 (52·8%) were female and 202 (47·2%) were male, while 190 (44·4%) were within the age range 5–10 years and 238 (55·6%) were within the age range 11–15 years (Table 3).
Table 3. Demography of study participant across public primary schools surveyed
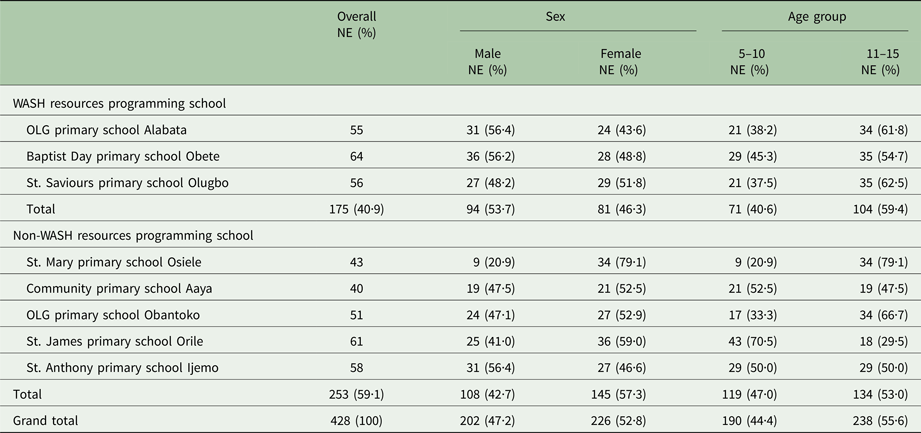
NE, Number examined.
Status and condition of safe water supply
All the WASH resources programming schools had functional hand pump boreholes. Two out of five (40·0%) of the non-WASH resource programming schools also had similar boreholes, though non-functioning. A cumulative score of 15 (100%) for safe water supply and conditions was recorded in WASH resource programming schools, whereas a cumulative score of 4 (16·0%) was recorded in the non-WASH resource programming schools. There was significant difference (P < 0·001) in safe water supply between the two categories of school (Table 4).
Table 4. Water supply conditions of Study schools and Cummulative Test Scores
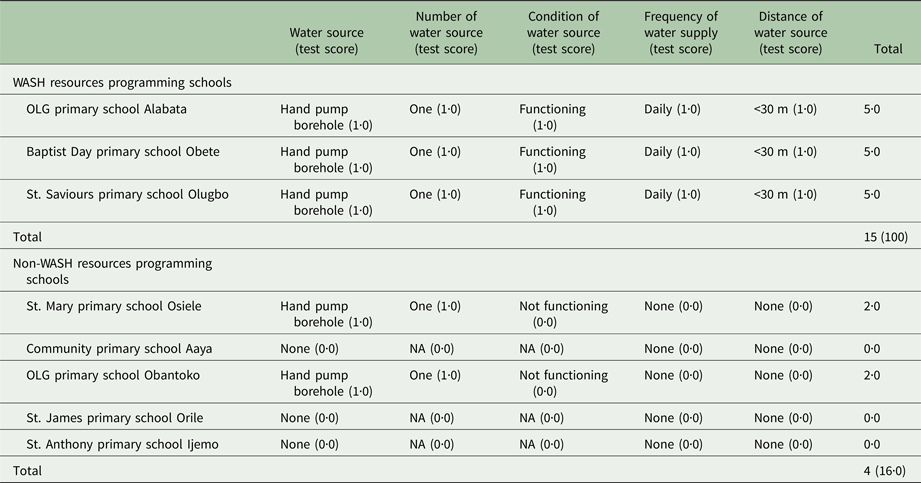
χ 2, df, P value = 10·8, 1, 0·000; Test score: 1·0, improved condition; 0·0, non-improved condition; NA, not applicable.
Status and condition of sanitation
A total of 38 toilets were assessed, 19 (50%) each in both categories. Of the 38 toilets, only 25 (66%) were in use by the children in all the schools; 11 (44·0%) belonging to WASH resource programming schools and 14 (56·0%) in non-resource programming schools. In all the schools, only 13 (34·2%) of the toilets were clean (without foul odour or feces around the pit-hole) and they all belonged to non-resource programming schools. Although all the toilets in WASH resource programming schools were in use, but they were very dirty. There was a water closet system in two of the non-WASH resource programming schools in a dirty condition. There was no provision for soap and water for hand washing after toilet usage in both categories of schools. All toilet facilities were communal and not segregated by gender in all schools. The recommended estimated ratio of toilets to pupils’ population were below WHO set standards (Table 5). A cumulative score of 8 (44·4%) was recorded for sanitation in the WASH resource programming schools, and a cumulative score of 6 (20·0%) was recorded in the non-WASH resource programming schools. The test scores for sanitation between WASH and non-WASH resource programming schools were not significantly different (P = 0·07).
Table 5. Sanitation condition of study schools and cumulative test scores
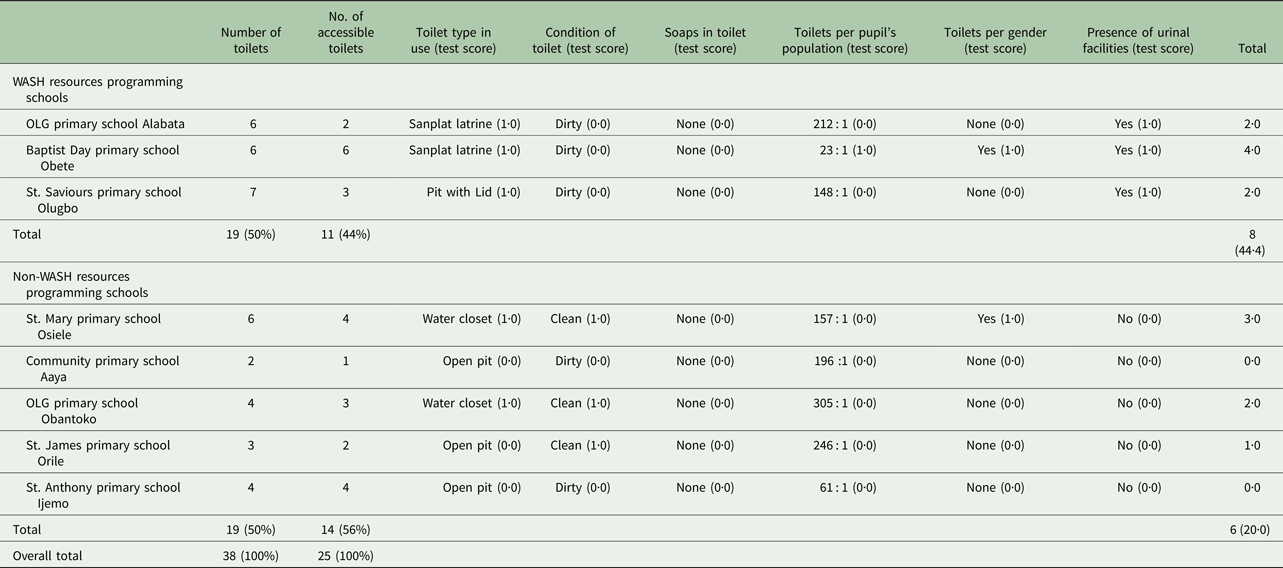
χ 2, df, P value = 3·3, 1, 0·07; Test score: 1·0, improved condition; 0·0, non-improved condition, NA, not applicable.
Status and condition of environmental hygiene resource
All the three WASH resource programming schools had garbage cans in their school premises. Whereas only one out of the five non-WASH resource programming school had garbage cans. Drinking water buckets with common drinking cups were absent in the classrooms of all schools surveyed (Table 6). A negative scoring method was employed under the item ‘usage of common cups’. This is simply because using common cups is not a good hygienic practice. So, if common cups were present in the classrooms, we deducted a point from their hygienic score (Table 6). The cumulative test score for the environmental hygiene of WASH resource programming schools was 11 (73·3%), while seven (26·9%) was recorded for non-WASH resources programming schools. There was a significant difference (P = 0·004) in the status and condition of environmental hygiene between the two categories.
Table 6. Environmental hygiene condition of study schools and cumulative test scores
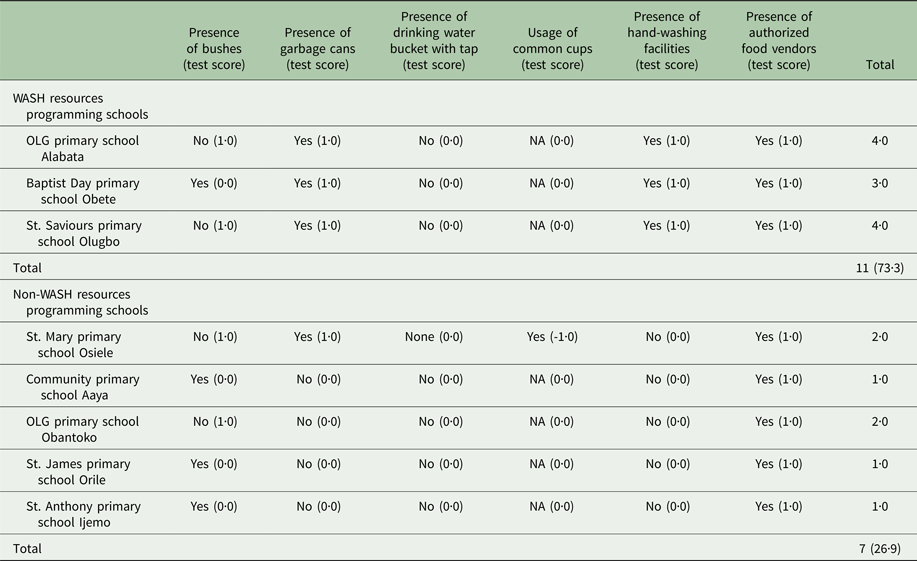
χ 2, df, P value = 8·3, 1, 0·004; Test score: 1·0, improved condition; 0·0, non-improved condition, NA, not applicable.
School of knowledge, attitudes and practices
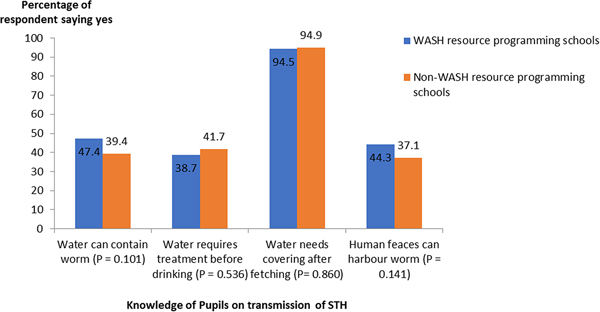
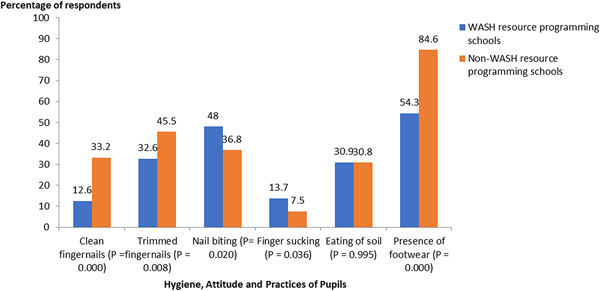
School children from non-WASH resource programming schools (n = 120, 47·4%) knew that human feces and water can harbour intestinal worms compared with children (n = 69, 39·4%) from WASH resource programming schools. However, this knowledge was not significantly different (P = 0·1) between groups (Fig. 2). Hygienic practices such as nail biting, finger sucking, pica and wearing of shoes differ in both group of schools. School children from non-WASH resources programming schools (n = 84, 33·2%) had more clean fingernails, which was significantly different (P < 0·001) in comparison with school children (n = 22, 12·6%) from WASH resource programming schools. Pupils with trimmed fingernails were significantly more in the non-WASH resource programming schools (n = 115, 45·5%) compared to WASH resources programming schools (n = 57, 32·6%) (P = 0·008) (Fig. 3). Pupils who Bite and suck fingernails from WASH resource programming schools (n = 84, 48·0%) were significantly (P = 0·04) more than those from non-WASH resource programming schools (n = 93, 36·8%). Wearing of footwear was also a more common feature among children from non-WASH resource programming (n = 214, 84·6%) compared with children from WASH resource programming schools (n = 95, 54·3%). There was a significant statistical difference between the use of footwear by children and school categories (P < 0·001) (Fig. 3).
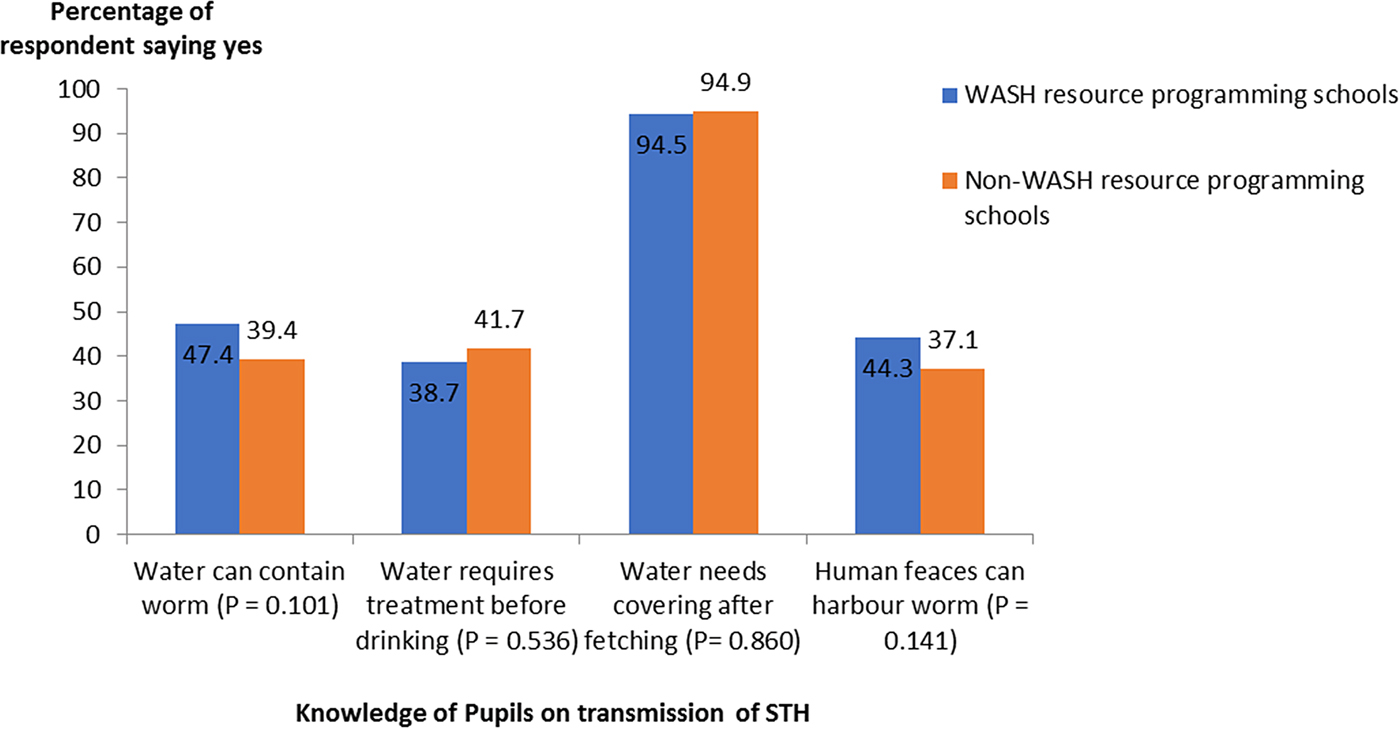
Fig. 2. Knowledge of study participants about transmission of STH infections.
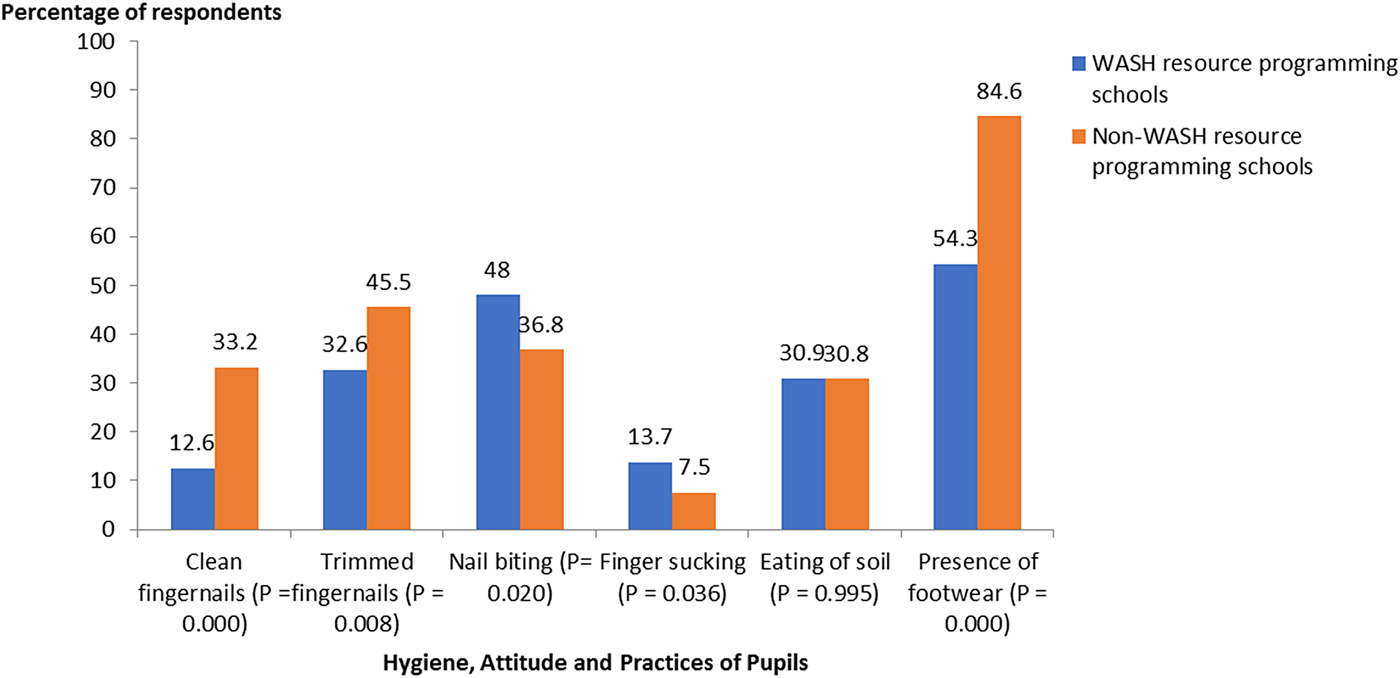
Fig. 3. Hygiene, attitudes and practices of study participant.
Prevalence and intensity of STH infections
A total of 143 (33·4%) of 428 children examined were infected with at least a species of STH. Prevalence of infection for hookworms, A. lumbricoides and T. trichiura was 26·2, 18·2 and 1·6%, respectively. Comparison of STH prevalence between the two categories of schools revealed 37·5% in non-WASH resource programming schools compared with 27·4% in WASH resource programming schools. There was a significant difference in STH prevalence between the categories of studied schools (P < 0·001) (Table 7).
Table 7. Prevalence of STH infections among the study participants
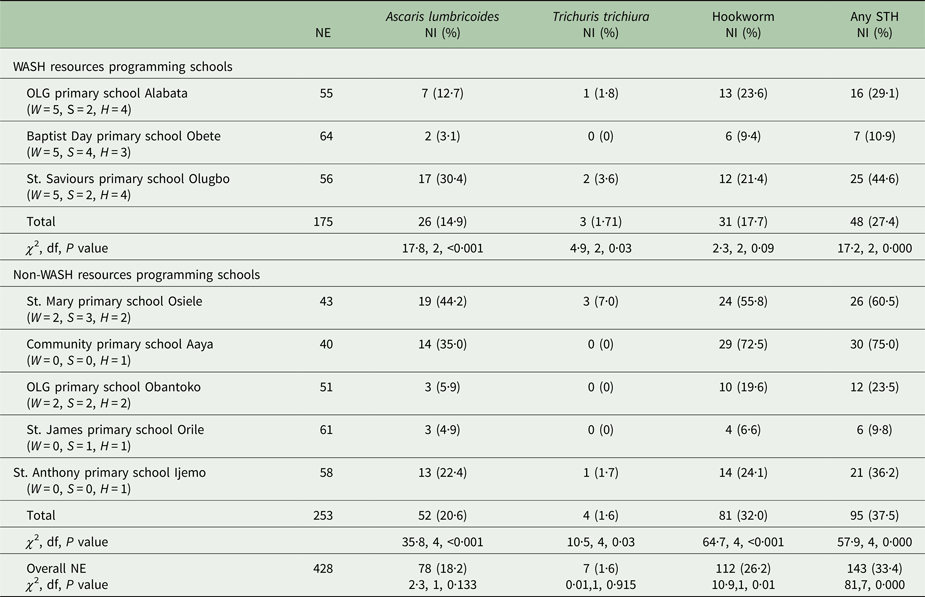
W, Water cumulative score; S, Sanitation cumulative score; H, Hygiene cumulative score; NE, Number examined; NI, Number infected.
Ascaris lumbricoides mean infection intensities were higher in WASH resource programming schools (0·7237), compared with non-WASH resource programming schools (0·5497), although there was no significant difference (P = 0·2). Intensities of Trichuriasis (0·1193) and hookworm (0·6097) were higher in non-WASH resource programming schools compared with WASH resource programming schools. A significant difference (P = 0·005) was recorded for the intensity of hookworm infection between the school categories (Table 8).
Table 8. Intensity of STH infections among the study participants
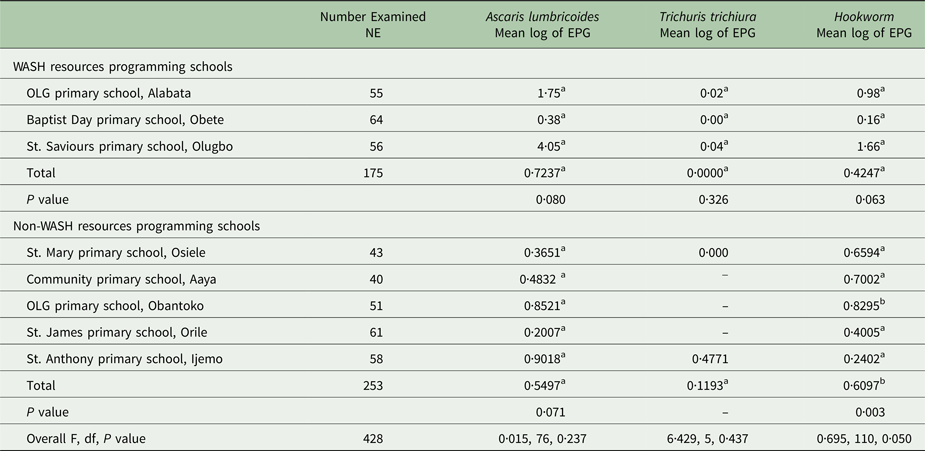
Mean values with same superscript across the same column are not significantly different at P < 0·05, EPG: egg per gram of feces.
Discussion
The overall prevalence reported for STH infection in this study reflects the status of helminthiasis morbidity in the study area, and reiterates the importance of complimenting school-based deworming activities with the provision of safe water, sanitation and hygiene resource (Freeman et al. Reference Freeman, Ogden, Jacobson, Abbott, Addiss, Amnie, Cairncross, Callejas, Colford, Emerson, Fenwick, Fishman, Gallo, Grimes, Karapetyan, Keene, Lammie, MacArthur, Lochery, Petach, Platt, Prabasi, Rosenboom, Roy, Saywell, Schechtman, Tantri, Velleman and Utzinger2013; Campbell et al. Reference Campbell, Savage, Gray, Atkinson, SoaresMagalhães, Nery, McCarthy, Velleman, Wicken, Traub, Williams, Andrews and Clements2014). The lower prevalence of STH infections observed among pupils from WASH resources programming schools compared with those from non-WASH resource programming schools, further provides evidence to support the ongoing discussion that WASH resources may have secondary influence on the transmission of STH (Freeman et al. Reference Freeman, Greene, Dreibelbis, Saboori, Muga, Brumback and Rheingans2012, Reference Freeman, Ogden, Jacobson, Abbott, Addiss, Amnie, Cairncross, Callejas, Colford, Emerson, Fenwick, Fishman, Gallo, Grimes, Karapetyan, Keene, Lammie, MacArthur, Lochery, Petach, Platt, Prabasi, Rosenboom, Roy, Saywell, Schechtman, Tantri, Velleman and Utzinger2013; Ziegelbauer et al. Reference Ziegelbauer, Speich, Mäusezahl, Bos, Keiser and Utzinger2012; Campbell et al. Reference Campbell, Savage, Gray, Atkinson, SoaresMagalhães, Nery, McCarthy, Velleman, Wicken, Traub, Williams, Andrews and Clements2014). However, STH prevalence levels in WASH resourced schools are still above mandatory treatment levels (i.e. 20%). In fact, the highest intensity estimate for STH infection was recorded in one of the WASH resource schools. This is an indication of incessant transmission despite the availability of WASH resource. It is therefore reasonable that reduction in the transmission of STH among SAC cannot be achieved or sustained only by providing WASH resources. Inadequate numbers of latrines, limited accessibility and lack of maintenance by school management have been documented as factors that predisposes pupils to defecation on open grounds and nearby bushes (Xuan et al. Reference Xuan, Hoat, Rheinlander, Dalsgaard and Konradsen2012). It is therefore important to take into cognizance, issues of adequacy and maintenance of WASH resources to ensure sustained and effective intervention effect on STH transmission and control.
Despite the recommendation of WHO on ‘1 toilet hole for 50 male or 30 female pupils, respectively, only one out of the eight schools surveyed met the above recommendation (Adams et al. Reference Adams, Bartram, Chartier and Sims2009). Also, not all available toilet facilities were accessible to the pupils, the clean and well-maintained ones were kept for the teachers. Segregating toilets per gender is therefore not feasible since toilets are inadequate and inaccessible to pupils. This indicates the need for more provision and maintenance of toilets in the public primary school's system. In addition, the consequences of poor sanitary conditions on hookworm transmission are well established; most especially in areas where wearing of footwear is not a common habit. Although the cumulative sanitation scores in the resourced and non-resourced schools appear to be equivalent, the non-WASH resourced schools had much greater percentage of children-wearing shoes (84%); yet, hookworm infection was much higher in non-resourced schools. Wearing of shoes by SAC is not a common habit in typical rural African communities. School children mostly wear shoes when they go to school and as well they pull them off while playing on school fields.
Water is a critical component of WASH resources. It is important for hand washing especially when soap is available. However, no soaps were seen in the schools surveyed. For non-WASH resource programming schools, water was not available in the school premises and pupils searched for water at locations outside the school premises, thus exposing themselves to infections transmitted through unsafe water sources (Lorna et al. Reference Lorna, Kaufmann, David, Wayne, Laurence and John2005). Lack of access to safe water may have contributed to the high burden of STH infections recorded among children attending non-intervention schools. Furthermore, the scarcity of water resource or its inadequate quantity in non-WASH resource programming schools may not meet the WHO recommendation of ‘at least 5 L of water’ per children in day-schools. This may be a factor promoting transmission of STH when children engage in poor hygienic practices such as nail biting and finger sucking after using the toilets.
Finally, majority of the pupils were ignorant of the fact that water and human feces can harbour helminth's eggs. In fact, children from WASH resources programming schools were not better off in terms of knowledge when compared with their counterpart in non-WASH schools. This observation was unexpected and may be an indicator of the lack of poor hygiene and sanitation education in WASH resource programming schools. The higher infection rates recorded in non-WASH resource programming schools despite their little knowledge about STH transmission reflects how insignificant the results of such knowledge could be on STH occurrence without WASH intervention. Effective hygiene education should be incorporated into the primary school curriculum, as this will help promote long-term positive behavioural changes among pupil both in school and at home (Jia et al. Reference Jia, Melville, Utzinger, King and Zhou2012; Nasr et al. Reference Nasr, Al-Mekhlafi, Ahmed, Roslan and Bulgiba2013).
Concluding remarks
Our study shows that there was a reduction in helminthiasis burden in schools benefitting from WASH resources compared with those schools not benefitting. However, these differences may also have been influenced by factors, such as the location of schools, soil type, precipitation, vegetation and temperature, which were not part of this study. Nevertheless, this study portrays the potentials of sustainable WASH resource programming implementation on reduction of morbidity and transmission of STH in public primary schools’.
Acknowledgements
We are grateful to the study participants who generously gave their time to be involved in this study.
Financial support
This work received financial support from TDR, the Special Programme for Research and Training in Tropical Diseases, co-sponsored by UNICEF, UNDP, the World Bank and WHO.
Conflict of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.