INTRODUCTION
Schistosomiasis is a disease of poverty, caused by infection with trematode parasites belonging to the genus Schistosoma (Colley et al. Reference Colley, Bustinduy, Secor and King2014). The disease is endemic in 75 countries, with over 200 million people affected and about 85% of these cases occurring in Africa (Steinmann et al. Reference Steinmann, Keiser, Bos, Tanner and Utzinger2006; WHO, 2010). In sub-Saharan Africa (SSA) there are two forms of schistosomiasis, intestinal and urogenital, each associated with different schistosome species (Colley et al. Reference Colley, Bustinduy, Secor and King2014). The most pervasive form, urogenital schistosomiasis, is caused by Schistosoma haematobium and is predominant in Africa, Middle East and Corsica (WHO, 2016). The disease has a classic sign of haematuria (blood in urine), often with underlying fibrosis of the bladder and ureter, and in more advanced cases kidney damage (WHO, 2016). Across West Africa, schistosomiasis can be common in rural areas and in an attempt to control morbidity; there are several ongoing national control programmes (NCPs). These programmes are chiefly engaged in preventive chemotherapy campaigns administering praziquantel en masse to targeted groups such as school-aged children (Lai et al. Reference Lai, Biedermann, Ekpo, Garba, Mathieu, Midzi, Mwinzi, N'Goran, Raso, Assare, Sacko, Schur, Talla, Tchuente, Toure, Winkler, Utzinger and Vounatsou2015; WHO, 2016).
Across SSA each form of schistosomiasis has a distribution, broadly tracking transmission foci where permissive freshwater intermediate snail hosts occur (Ekpo et al. Reference Ekpo, Huerlimann, Schur, Oluwole, Abe, Mafe, Nebe, Isiyaku, Olamiju, Kadiri, Poopola, Braide, Saka, Mafiana, Kristensen, Utzinger and Vounatsou2013; Lai et al. Reference Lai, Biedermann, Ekpo, Garba, Mathieu, Midzi, Mwinzi, N'Goran, Raso, Assare, Sacko, Schur, Talla, Tchuente, Toure, Winkler, Utzinger and Vounatsou2015). In terms of urogenital schistosomiasis, Nigeria has one of the highest burdens in West Africa, with the disease endemic across all 36 States in the country. Around certain transmission foci in Ogun State, for example, local prevalence can very high ~90% (Akinwale et al. Reference Akinwale, Ajayi, Akande, Gyang, Adeleke, AK Adeneye, MO Adebayo and Dike2010) as water contact activities among the rural populace, albeit recreational and or domestic, are particularly common. These typically include: bathing, swimming, drinking, washing clothes or kitchen utensils and fetching of water, such that each activity serves as an often-daily pathway for acquiring infections (Ekpo et al. Reference Ekpo, Mafiana, Adeofun, Solarin and Idowu2008, Reference Ekpo, Laja-Deile, Oluwole, Sam-Wobo and Mafiana2010, Reference Ekpo, Huerlimann, Schur, Oluwole, Abe, Mafe, Nebe, Isiyaku, Olamiju, Kadiri, Poopola, Braide, Saka, Mafiana, Kristensen, Utzinger and Vounatsou2013; Sam-Wobo et al. Reference Sam-Wobo, Ekpo, Ameh and Osileye2009; Oladoyin et al. Reference Oladoyin, Akindele and Uchendu2015).
With its predilection to colonize the lower pelvic venous system, especially the vesical plexus, eggs of S. haematobium are typically voided in urine and detected by urine microscopy (Stothard et al. Reference Stothard, Stanton, Bustinduy, Sousa-Figueiredo, Van Dam, Betson, Waterhouse, Ward, Allan, Hassan, Al-Helal, Memish and Rollinson2014). Their exit causes micro-perforations in the bladder wall with concomitant passage of venous blood, either in visible (macrohaematuria) or invisible (microhaematuria) amounts, the latter detectable by reagent strips (Stothard et al. Reference Stothard, Stanton, Bustinduy, Sousa-Figueiredo, Van Dam, Betson, Waterhouse, Ward, Allan, Hassan, Al-Helal, Memish and Rollinson2014). Those eggs that fail to exit the body become trapped, trigger an immuno-inflammatory reaction and elicit ‘classic’ lower/upper urinary pathology as well as progressive damage in other internal organs (Colley et al. Reference Colley, Bustinduy, Secor and King2014; Dawaki et al. Reference Dawaki, Al-Mekhlafi, Ithoi, Ibrahim, Abdulsalam, Ahmed, Sady, Nasr and Atroosh2015). Whilst blood in urine is a cardinal sign of urogenital schistosomiasis; the condition may be so common in afflicted communities such that individuals accept and downplay its local clinical significance and largely ignore other sub-clinical morbidities. To better describe these disease states, advanced imagery, e.g. ultrasonography or semi-invasive methods, e.g. colposcopy is needed to visualize disease especially that of the lower genital tract (Kjetland et al. Reference Kjetland, Leutscher and Ndhlovu2012; Holmen et al. Reference Holmen, Onsrud, Vennervald, Albregtsen, Taylor, Moodley, van Lieshout, Pillay, Lillebo, Kleppa and Kjetland2014). Since such techniques are not available in primary health units, the manifestations of genital schistosomiasis go unnoticed whilst clinical symptoms may, for example, be attributed to sexually transmitted infections (STIs). Indeed, an incomplete surveillance and under-reporting of S. haematobium infection curtails the management of urogenital schistosomiasis in many rural parts of Africa (Stothard et al. Reference Stothard, Stanton, Bustinduy, Sousa-Figueiredo, Van Dam, Betson, Waterhouse, Ward, Allan, Hassan, Al-Helal, Memish and Rollinson2014).
Upon migration within the tributaries of the internal iliac vein, adult S. haematobium eggs colonize other parts of the venous pelvic system causing damage to the reproductive organs and genitalia of both genders (Colley et al. Reference Colley, Bustinduy, Secor and King2014; Kjetland et al. Reference Kjetland, Norseth, Taylor, Lillebo, Kleppa, Holmen, Andebirhan, Yohannes, Gundersen, Vennervald, Bagratee, Onsrud and Leutscher2014; Stecher et al. Reference Stecher, Kallestrup, Kjetland, Vennervald and Petersen2015; WHO, 2015). For example, schistosome eggs lodged in the uterine and vaginal venous plexus cause female genital schistosomiasis (FGS) which is a major detriment to women's health in SSA (HellingGiese et al. Reference HellingGiese, Kjetland, Gundersen, Poggensee, Richter, Krantz and Feldmeier1996a , Reference HellingGiese, Sjaastad, Poggensee, Kjetland, Richter, Chitsulo, Kumwenda, Racz, Roald, Gundersen, Krantz and Feldmeier b ; Kjetland et al. Reference Kjetland, Kurewa, Ndhlovu, Midzi, Gwanzura, Mason, Gomo, Sandvik, Mduluza, Friis and Gundersen2008; Christinet et al. Reference Christinet, Lazdins-Helds, Stothard and Reinhard-Rupp2016). Although FGS was specifically recognized over 100 years ago, its pathology often mimics sexually transmitted diseases and is often incorrectly reported (Christinet et al. Reference Christinet, Lazdins-Helds, Stothard and Reinhard-Rupp2016). More recently, the importance of FGS in East, Central and Southern African (Poggensee et al. Reference Poggensee, Kiwelu, Saria, Righter, Krantz and Feldmeier1998, Reference Poggensee, Sahebali, Van Marck, Swai, Krantz and Feldmeier2001; Poggensee and Feldmeier, Reference Poggensee and Feldmeier2001; Kjetland et al. Reference Kjetland, Kurewa, Ndhlovu, Midzi, Gwanzura, Mason, Gomo, Sandvik, Mduluza, Friis and Gundersen2008, Reference Kjetland, Leutscher and Ndhlovu2012; Downs et al. Reference Downs, Mguta, Kaatano, Mitchell, Bang, Simplice, Kalluvya, Changalucha, Johnson and Fitzgerald2011, Reference Downs, Kabangila, Verweij, Jaka, Peck, Kalluvya, Changalucha, Johnson, van Lieshout and Fitzgerald2013; Randrianasolo et al. Reference Randrianasolo, Jourdan, Ravoniarimbinina, Ramarokoto, Rakotomanana, Ravaoalimalala, Gundersen, Feldmeier, Vennervald, van Lieshout, Roald, Leutscher and Kjetland2015) has been much raised following several seminal studies leading to production and use of the FGS pocket colour atlas, endorsed by the WHO (Kjetland et al. Reference Kjetland, Norseth, Taylor, Lillebo, Kleppa, Holmen, Andebirhan, Yohannes, Gundersen, Vennervald, Bagratee, Onsrud and Leutscher2014; Norseth et al. Reference Norseth, Ndhlovu, Kleppa, Randrianasolo, Jourdan, Roald, Holmen, Gundersen, Bagratee, Onsrud and Kjetland2014; WHO, 2015). The FGS pocket atlas provides a useful reference guide to describe the disease upon colposcopy, intending to empower disease surveillance systems to observe and report this condition.
In Nigeria, the geographical distribution of S. haematobium is extensive, and the NCP is attempting to establish large-scale control activities in several disease-endemic states (Ekpo et al. Reference Ekpo, Huerlimann, Schur, Oluwole, Abe, Mafe, Nebe, Isiyaku, Olamiju, Kadiri, Poopola, Braide, Saka, Mafiana, Kristensen, Utzinger and Vounatsou2013). However, there are several states where preventive chemotherapy has not yet been rolled out, and more generally there are no specific actions on FGS in any areas. Here, we report on the findings of a pilot survey for FGS undertaken in Ogun State where S. haematobium is endemic, and no formal control programme currently exists.
MATERIALS AND METHODS
Study area and subjects
The epidemiological and clinical survey was undertaken in four villages of Abule-Titun, Apojola, Ibaro and Imala-Odo, near Abeokuta, Ogun State. All villages are located on the immediate shoreline of Oyan River dam (7°15′30″ N, 3°15′20″ Ε), and were known to be highly endemic since 1991 till date (Ofoezie et al. Reference Ofoezie, Imevbore, Balogun, Ogunkoya and Asalu1991; Mafiana et al. Reference Mafiana, Ekpo and Ojo2003; Akinwale et al. Reference Akinwale, Ajayi, Akande, Gyang, Adeleke, AK Adeneye, MO Adebayo and Dike2010; Ekpo et al. Reference Ekpo, Fafunwa, Oluwole, Abe and Mafiana2012). All resident young girls (>5–16 years old) and women of child bearing age (>16–49 years old) were invited to participate in the study. The communities (average size of 200 inhabitants) are predominantly fishing settlements established soon after the construction of the Oyan River dam (Ofoezie et al. Reference Ofoezie, Imevbore, Balogun, Ogunkoya and Asalu1991). Locally there is an annual school-based treatment campaign in school children, but infections in pre-school children and non-enrolled school-aged children are not treated. Similarly, the Ogun State does not provide access to preventive treatment for adults although praziquantel can be purchased in nearby private pharmacies at a cost of 1 USD per tablet.
Ethical approval and considerations
Ethical approval was granted by the ethical review board of State Hospital Ijaye, and Ogun State Ministry of Health, Abeokuta. Community sensitization and mobilization was initially undertaken through the primary health care coordinator and local headman/community leader in each village. After explaining the objectives of the study informed written consent was obtained from the child's guardian and adult participant. All participant consenting to the study procedures were provided with praziquantel treatment (40 mg kg−1) in accordance with their height, using dosing poles.
Participant interviews and questionnaires
Participant interview was conducted on site by the field team. A questionnaire was used to obtain information on socio-demographic characteristics, and each participant was asked to describe any urogenital signs and symptoms they were experiencing currently or recently. In an attempt to standardize symptomologies a vaginal discharge colour chart (Fig. 1) was use used to clarify discussions (Hegertun et al. Reference Hegertun, Sulheim Gundersen, Kleppa, Zulu, Gundersen, Taylor, Kvalsvig and Kjetland2013). The colour chart exemplifies presence of blood or vaginal discharge. Environmental risk factors such as participants’ day-to-day water contact activities putatively associated with urinary schistosomiasis and FGS were ascertained through more in-depth interviews. All discussions were made in local languages and simplified to the best understanding of the young female children (5–15 years).
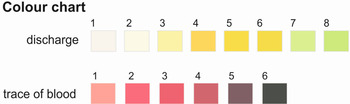
Fig. 1. Standardized symptomologies using a vaginal discharge colour chart (Hegertun et al. Reference Hegertun, Sulheim Gundersen, Kleppa, Zulu, Gundersen, Taylor, Kvalsvig and Kjetland2013) for visual comparisons.
Diagnosis of urogenital schistosomiasis
A 10 mL mid-stream urine sample was collected from each participant during late morning or early afternoon. Urines were preserved with 10% formaldehyde and transported to the laboratory chilled on ice-packs for light microscopy. Samples were centrifuged at 1500 rpm for 10 min in a benchtop centrifuge, and sediments were examined for ova of S. haematobium at ×100 magnification. Sample processing and examination, as well as clinical investigations and colposcopy, were undertaken by a female gynaecologist in the local community health facility.
Colposcopy and clinical examination for FGS
Twenty adult female participants out of the 317 participants consented to gynaecological examination by a female gynaecologist. Examination commenced with vaginal lavage with a solution of 3% sodium chloride then followed by photo-colposcopy. The cervico-vaginal surfaces were inspected following guidelines in the outlined in the WHO FGS pocket atlas, specifically noting five clinically visible manifestations: contact bleeding, pre-contact bleeding, yellow sandy patches, grainy sandy patches and abnormal blood vessels (WHO, 2015). FGS was defined as having grainy sandy patches or homogenous yellow sandy patches. Tissue biopsy of the cervico-vaginal surface was not performed.
Data tabulations and statistical analysis
Raw data were entered into Excel database and then analysed using SPSS IBM 20.0 version, Armonk, NY, IBM Corp. Data were first subjected to descriptive statistics, including frequencies and cross-tabulations, followed by Pearson Chi-square analysis to test for variables that were significantly associated with urogenital schistosomiasis.
RESULTS
Demographic status of participants
A total of 317 participants, 187 girls and 130 adult women were recruited into the study with 64 (20·2%) from Ibaro; 131 (41·3%) from Imala-Odo; 69 (21·8%) from Abule-Titun and 53 (16·7%) from Apojola communities. Amongst the adults, almost all were married, and only one person was separated. 65·6% (208/317) of the participants had primary education (Table 1).
Table 1. Demographic characteristics of participants

Urogenital schistosomiasis and water contact behaviours
The overall prevalence of urogenital schistosomiasis was 47·0% (149/317) with an overall geometric mean egg intensity (GMI) of 0·77 eggs per 10 mL (Table 2). There was a significant difference (P < 0·05) between the prevalence and intensity of urogenital schistosomiasis by age categories of participants. Prevalence and intensity was significantly (P < 0·05) higher among children aged 5–15 years (64·7%) with GMI of 0·95 ± 0·56 eggs per 10 mL of urine than young adult women aged 16–25 years 29·0% with GMI of 0·42 ± 0·86 eggs per 10 mL of urine and adult women aged 26–49 years 14·7% with GMI of 0·19 ± 0·59 eggs per 10 mL. By village, significant differences (P < 0·05) were found between mean intensities recorded across the four communities surveyed. In terms of water contact, 294 (92·7%) fetched water every day from the dam, 166 (52·4%) engaged in fishing activities, 296 (93·4%) washed clothes at the dam, while 306 (96·5%) bathed or swam in the dam.
Table 2. Prevalence and Geometric Mean Intensities of urinary schistosomiasis across demographic characteristics of participants
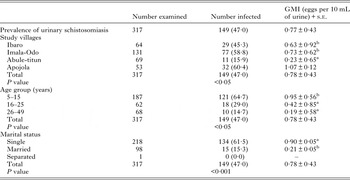
GMI, Geometric Mean Intensity (mean values with the same superscript are not significantly different at P < 0·05).
Self-reported symptoms and colposcopy
Female participants reported symptoms such as blood in urine, itching of the vaginal, burning sensation in the genital area, swelling/lump, stress incontinence, urge incontinence and dysuria. Only two symptoms (haematuria and vaginal discharge) showed significance across the age categories. Female participants aged 5–15 years had the highest morbidity prevalence for haematuria, while those aged 26–49 years had the highest morbidity prevalence for vaginal discharge (Table 3). Of the 87 (27·4%) respondents that recalled having virginal discharges, only 27 (31·0%) could recall the colour of the vaginal discharge they had experienced in the past or were currently experiencing with the majority 11 (40·7%) recalling a yellow vaginal colour discharge. Other colours forms of discharges were in form of brown, 9 (33·3%); red, 5 (18·5%), black, 1 (3·7%) and butter, 1 (3·7%) colours, respectively.
Table 3. Self-reported symptoms and association with egg-patent urinary schistosomiasis
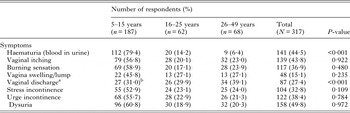
a The vaginal discharge was classified against colour reference chart FGS with associated severity risk grade (Fig. 1).
b 11 yellow discharges (graded 8+), 9 brown discharges (graded 5+), 5 red discharges (graded 2+), 1 black discharge (graded 8+) and 1 butter discharge (graded 4+).
Twenty women out of the 317 study participants (6·3%) consented to gynaecological and a photo-colposcopic examinations (Table 4). Upon examination 14 (70·0%) had vaginal or cervical anomalies (Fig. 2) that could be classified according to the WHO FGS pocket atlas (Table 4). The symptomatic profile of females presenting FGS pathology includes dysuria (57·1%), vaginal itching (42·6%), vaginal swelling (28·6%) and burning sensation (28·6%) is shown in (Table 5), while the profile and FGS pathology among the 20 women who consented to gynaecological examination is presented in Table 6.
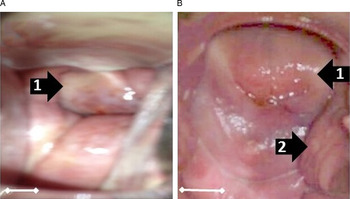
Fig. 2. Vaginal or cervical anomalies seen in FGS cases. Images of cervix taken using colposcopy. (A) Grainy sandy patches (1) and homogenous yellow sandy patches around the cervical wall; (B) Sandy patches: (1) and rubbery papules (2) and homogenous yellow sandy patches around the cervical wall (Scale bar indicates 1 cm).
Table 4. Occurrence and clinical presentation of FGS within the 20 women examined
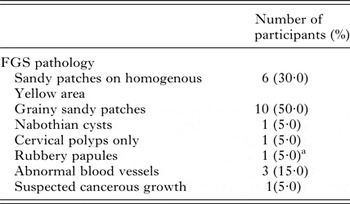
a This is the first time this condition has been reported outside of Madagascar (Randrianasolo et al. Reference Randrianasolo, Jourdan, Ravoniarimbinina, Ramarokoto, Rakotomanana, Ravaoalimalala, Gundersen, Feldmeier, Vennervald, van Lieshout, Roald, Leutscher and Kjetland2015).
Table 5. Reported symptoms in women presenting with FGS
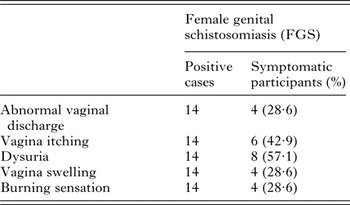
Table 6. Profile and FGS pathology among the 20 women consenting to gynaecological examination

DISCUSSION
Among the females, with urogenital schistosomiasis, one third reported to have vaginal discharge and just under half of the study participants had S. haematobium eggs visible in the urine indicative of active urogenital schistosomiasis. As only a single urine sample was taken the actual prevalence of active as well as past infection with urogenital schistosomiasis is likely higher, for in the light of daily water contact activities undertaken in these villages, such as swimming, bathing, washing clothes, fishing, etc., the risk here is universal. Typical of the age-prevalence relationship for urinary schistosomiasis, egg-patent prevalence was higher in the 5–15-year-old category. Nonetheless, some of the older girls and adult women were excreting eggs in urine. Whilst there have been several reports describing prevalence and intensity of urogenital schistosomiasis along this shoreline, and in Ogun State in general (Mafiana et al. Reference Mafiana, Ekpo and Ojo2003; Ekpo et al. Reference Ekpo, Mafiana, Adeofun, Solarin and Idowu2008, Reference Ekpo, Laja-Deile, Oluwole, Sam-Wobo and Mafiana2010; Akinwale et al. Reference Akinwale, Ajayi, Akande, Gyang, Adeleke, AK Adeneye, MO Adebayo and Dike2010), there has been no prior attempt to document the occurrence of FGS here or elsewhere in Nigeria.
Of the women that volunteered for genital examination, we found that more than half had clinical manifestations of FGS. It is remarkable that such a locally pervasive disease, which is of obvious detriment to the health of women has not attracted more attention, although like elsewhere in Nigeria many perhaps suffer in silence (Dawaki et al. Reference Dawaki, Al-Mekhlafi, Ithoi, Ibrahim, Abdulsalam, Ahmed, Sady, Nasr and Atroosh2015). Surveillance for FGS has been totally overlooked, whilst the disease has been firmly engrained in these communicates for decades, first formally reported in 1991 (Ofoezie et al. Reference Ofoezie, Imevbore, Balogun, Ogunkoya and Asalu1991). More broadly, clinical surveillance of FGS in West Africa has not been undertaken largely due to lack of awareness and inexperience of health personnel at all cadres (Christinet et al. Reference Christinet, Lazdins-Helds, Stothard and Reinhard-Rupp2016; Holmen et al. Reference Holmen, Galappaththi-Arachchige, Kleppa, Pillay, Naicker, Taylor, Onsrud, Kjetland and Albregtsen2016), inadequate resources for its detection within the primary care setting (WHO, 2015), as well as competing interests and priorities within NCPs that tackle more obvious issues associated with administration of preventive chemotherapy. The latter is an unfortunate polarity which on the one hand monitors and treats urinary signs and symptoms of schistosomiasis yet on the other totally neglects genital tract involvement.
To address this and building on seminal work in central and southern Africa, the production of the WHO FGS pocket atlas has attempted to spearhead concerted action against this FGS (Christinet et al. Reference Christinet, Lazdins-Helds, Stothard and Reinhard-Rupp2016). This is by encouraging both epidemiological and clinical studies; from an epidemiological perspective, introducing the use of simple symptomology questionnaires is eminently feasible in resource constrained settings and has shown to be informative (Kjetland et al. Reference Kjetland, Kurewa, Ndhlovu, Midzi, Gwanzura, Mason, Gomo, Sandvik, Mduluza, Friis and Gundersen2008; Holmen et al. Reference Holmen, Onsrud, Vennervald, Albregtsen, Taylor, Moodley, van Lieshout, Pillay, Lillebo, Kleppa and Kjetland2014). Where further clinical resources are available, the colour plate illustrations of pathognomonic lesions help to identify and classify vagino-cervical pathologies (Norseth et al. Reference Norseth, Ndhlovu, Kleppa, Randrianasolo, Jourdan, Roald, Holmen, Gundersen, Bagratee, Onsrud and Kjetland2014). Table 3 shows that a variety of symptoms were reported, the most significant of which appeared to be abnormal vaginal discharge. This was further categorized by making use of a simple visual colour chart for comparison (Hegertun et al. Reference Hegertun, Sulheim Gundersen, Kleppa, Zulu, Gundersen, Taylor, Kvalsvig and Kjetland2013). Similarly, Table 4 shows by colposcopy, a total of 70·0% of women had patent genital tract disease, which could be graded into typical FGS lesions, e.g. sandy patches and homogenous yellow areas. This level, although high is not surprising in light of FGS, reported in other parts of Africa (Kjetland et al. Reference Kjetland, Leutscher and Ndhlovu2012, Reference Kjetland, Norseth, Taylor, Lillebo, Kleppa, Holmen, Andebirhan, Yohannes, Gundersen, Vennervald, Bagratee, Onsrud and Leutscher2014). Two weakness of our study, however, was that firstly, we made no attempt to investigate other sexually transmitted infections that may cause similar symptoms as that of FGS and secondly, only women with active urogenital schistosomiasis consented and underwent colposcopic procedures. Thus, there probably remains an unknown proportion of women who may have FGS without active Schistosoma egg-excretion in the urine. This should be investigated in greater detail as it is well known that FGS is not always predicted by urine-egg patency (Poggensee et al. Reference Poggensee, Kiwelu, Saria, Righter, Krantz and Feldmeier1998; Holmen et al. Reference Holmen, Onsrud, Vennervald, Albregtsen, Taylor, Moodley, van Lieshout, Pillay, Lillebo, Kleppa and Kjetland2014).
Conducting colposcopy is certainly a critical step in detection and classification of clinical FGS as presented by visual lesions on the surfaces of the vagina and cervix. Where colposcopy is not possible, there should be alternative attempts to detect and confirm FGS (Randrianasolo et al. Reference Randrianasolo, Jourdan, Ravoniarimbinina, Ramarokoto, Rakotomanana, Ravaoalimalala, Gundersen, Feldmeier, Vennervald, van Lieshout, Roald, Leutscher and Kjetland2015), especially by application of more sensitive diagnostic methods that detect schistosome eggs or biomarker thereof before more serious lesion develop in the genitalia (Pillay et al. Reference Pillay, Taylor, Zulu, Gundersen, Kleppa, Lillebo, Kjetland, Brienen and van Lieshout2014, Reference Pillay, van Lieshout, Taylor, Sebitloane, Zulu, Kleppa, Roald and Kjetland2016). For example, if biopsy material or vaginal lavage fluid was more readily available then both histology and molecular DNA approaches could be used (Randrianasolo et al. Reference Randrianasolo, Jourdan, Ravoniarimbinina, Ramarokoto, Rakotomanana, Ravaoalimalala, Gundersen, Feldmeier, Vennervald, van Lieshout, Roald, Leutscher and Kjetland2015). The latter would only be possible if the eggs are viable, whereas calcified eggs would not excrete DNA. With promotion of the Sustainable Development Goals, there will be greater attention placed on women's well-being, which in part will focus upon reducing cervical cancers and increased screening for human papilloma virus (HPV) (Coleman et al. Reference Coleman, Cespedes, Cu-Uvin, Kosgei, Maloba, Anderson, Wilkin, Jaquet, Bohlius, Anastos and Wools-Kaloustian2016). It is evident that where urinary schistosomiasis is co-endemic the health system should integrate detection of FGS within such wider surveillance. By that token, praziquantel treatment should be made available to all women who are shown to have urogenital schistosomiasis or are at-risk of acquiring it in childhood (Bustinduy et al. Reference Bustinduy, Friedman, Kjetland, Ezeamama, Kabatereine, Stothard and King2016). We, therefore, encourage the Nigerian NCP to expand future access of praziquantel to all women at-risk and orchestrate an appropriate response to the challenge that FGS poses not only to detect it, but also prevent it.
Conclusion
Our pilot study has confirmed the occurrence of FGS in Ogun State, Nigeria and highlights that a substantive proportion of women with urogenital schistosomiasis have underlying gynaecological disease that is currently overlooked. We encourage the Nigerian NCP to develop a specific action plan against FGS and orchestrate an appropriate response not only to detect it, but also prevent it. Otherwise, millions of African women will continue to suffer unduly.
ACKNOWLEDGEMENTS
We are grateful to the study participants who generously gave their time to be involved in this study. We thank Dr Eyrun Kjetland for advice on colposcopy and providing FGS standardized vaginal discharge colour chart. Professor J. R. Stothard is Director of COUNTDOWN, an implementation research consortium that receives support from DFID, UK to support scale-up of interventions against neglected tropical diseases in Nigeria.
FINANCIAL SUPPORT
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
CONFLICT OF INTEREST
None.
ETHICAL STANDARDS
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008












