Introduction
Habitat destruction or alteration is considered the main cause of biodiversity loss (Laurence & Useche, Reference Laurence and Useche2009) and, because of the high rate of such destruction, it is necessary to evaluate management priorities for the conservation of ecological processes and biological complexity (Balmford et al., Reference Balmford, Crane, Dobson, Green and Mace2005). In the case of species associated with aquatic environments, isolation resulting from the loss of interconnected aquatic habitats is one of the most significant factors influencing their decline (Finn et al., Reference Finn, Bogan and Lytle2009; Griffiths et al., Reference Griffiths, Sewell and McCrea2010). It is therefore important to evaluate the ability of temporary or small ponds to meet the breeding, resting or feeding needs of species associated with aquatic environments (Brainwood & Burgin, Reference Brainwood and Burgin2009; Pinto-Cruz et al., Reference Pinto-Cruz, Molina, Barbour, Silva and Espírito-Santo2009). Unlike large water bodies, the role of small water bodies as hotspots has not been evaluated until recently (Gómez-Rodríguez et al., Reference Gómez-Rodríguez, Díaz-Paniagua, Serrano, Florencio and Portheault2009). In the Northern Hemisphere, although the number of natural ponds and lagoons is declining (Gallego-Fernández et al., Reference Gallego-Fernández, García-Mora and García-Novo1999), the availability of man-made water bodies has increased but they are generally subject to a high degree of various types of disturbance (Oertli et al., Reference Oertli, Céréghino, Hull and Miracle2009).
Man-made ponds are created in the Mediterranean basin mainly to supply the needs of wild ungulates and livestock and for irrigated agriculture. These ponds have different characteristics depending on their use, and their ecology varies depending on the intensity of use, the species present, human pressure, and the nature of the surrounding areas (Declerck et al., Reference Declerck, De Bie, Ercken, Hampel, Schrijvers and Van Wichelen2006). Man-made ponds are designed and managed mostly from an economic point of view and do not take into account environmental criteria. There is a lack of detailed information of the requirements of secondary consumer species at forest ponds but such information is more widely available for agricultural ponds (Paracuellos et al., Reference Paracuellos, Castro, Nevado, Oña, Matamala, García and Salas2002; Sebastián-González et al., Reference Sebastián-González, Sánchez-Zapata and Botella2010).
One way to assess habitats of high conservation priority is to determine the presence of threatened, key or umbrella species (Suter et al., Reference Suter, Graf and Hess2002; Larsen et al., Reference Larsen, Bladt and Rahbek2007; Delibes-Mateos et al., Reference Delibes-Mateos, Delibes, Ferreras and Villafuerte2008). This is based on the principle that ecosystem protection can be optimized by conservation of the ecological requirements of these species (Lambeck, Reference Lambeck1997). Among these requirements obtaining sufficient, suitable food is crucial and largely depends on the availability, abundance and quality of food resources (Newton, Reference Newton1998; Begon et al., Reference Begon, Harper and Townsend1999). Adaptive mechanisms such as skill, experience and plasticity to resource variation are developed by some umbrella species to avoid negative affects on population parameters (Costillo et al., Reference Costillo, Corbacho, Morán and Villegas2007).
The black stork Ciconia nigra is a long-distance migratory species whose breeding distribution includes the Palaearctic and southern Africa (Del Hoyo et al., Reference Del Hoyo, Elliot and Sargatal1992). The western limit of its range is in the Iberian Peninsula, where the breeding population is estimated to be 405–483 pairs (Cano et al., Reference Cano, Franco, Pacheco, Reis, Rosa and Fernández2006). Although the black stork is categorized as Least Concern globally (BirdLife International 2009) it is considered as Vulnerable in Portugal and Spain (Almeida et al., Reference Almeida, Catry, Encarnaçao, Franco, Granadeiro, Lopes, Cabral, Almeida, Almeida, Dellinger, Ferrand de Almeida and Oliveira2005; Ministry of the Environment, Rural and Marine Affairs, 2009). The species is considered an umbrella species (Lambeck, Reference Lambeck1997; Fleishman et al., Reference Fleishman, Blair and Murphy2001; Roberge & Angelstam, Reference Roberge and Angelstam2004) because of its large foraging range (Jiguet & Villarrubias, Reference Jiguet and Villarrubias2004) and the specificity of its requirements for food and nesting sites (Lôhmus & Sellis, Reference Lôhmus and Sellis2003; Seddon & Leech, Reference Seddon and Leech2008). The black stork’s habitat is in areas with high conservation status that are also important for many other species (Simberloff, Reference Simberloff1998; Vlachos et al., Reference Vlachos, Bakaloudis, Alexandrou, Bontzorlos and Papakosta2008), some of which are endemic to the Mediterranean region (Elvira, Reference Elvira1995; Rodríguez-Prieto & Fernández-Juricic, Reference Rodríguez-Prieto and Fernández-Juricic2004; Bernardos et al., Reference Bernardos, García-Barriuso, Sánchez-Anta and Amich2007). The black stork is a secondary consumer in the trophic chain, requiring invertebrate, amphibian, reptile and fish prey in various types of wetlands and grasslands (Ferrero & Pizarro, Reference Ferrero and Pizarro2003; Hampl et al., Reference Hampl, Bures, Baláz, Bobek and Pojer2005). The species is sensitive to habitat alteration and is particularly affected by human disturbances, such that its protection requires the promotion of best management practices for the environment on which it depends (Rosenvald & Lôhmus, Reference Rosenvald and Lôhmus2003) and the maintenance of a broad network of land of favourable conservation status (BirdLife International, 2004).
Detailed knowledge of a species’ selection of foraging habitat is essential for designing habitat management for conservation purposes (Manly et al., Reference Manly, MacDonald and Thomas1993; Zuberogoitia et al., Reference Zuberogoitia, Martínez, Martínez, Zabala, Calvo and Castillo2006; Paiva et al., Reference Paiva, Ramos, Martins, Almeida and Carvalho2007). For the black stork several studies have indicated that calm stretches of streams and rivers, natural or man-made ponds, and reservoir tails near forested areas are the species’ main feeding sites during the breeding season (Schneider-Jacoby, Reference Schneider-Jacoby1999; Ferrero & Pizarro, Reference Ferrero and Pizarro2003). However, research has not addressed the variables contributing to this species’ selection of feeding locations.
This study aimed to determine the characteristics of ponds and their surrounding areas that influence selection of feeding habitat by the black stork. Our goal is to recommend best management practices in pond construction or restoration to improve the conservation status of the communities inhabiting these aquatic hotspots.
Study area
We studied 85 ponds in central and western Spain (Fig. 1) on 21 privately owned estates in seven Special Protected Areas. We first inventoried and determined the location of wetlands on the estates and then selected five natural and 80 man-made ponds. The area includes forests with Mediterranean scrub and an agrosilvopastoral mosaic, dominated by tree species of the genus Quercus (Q. rotundifolia and Q. suber) and a shrub layer (principally Cistus ladanifer, Genista sp. and Erica sp.).
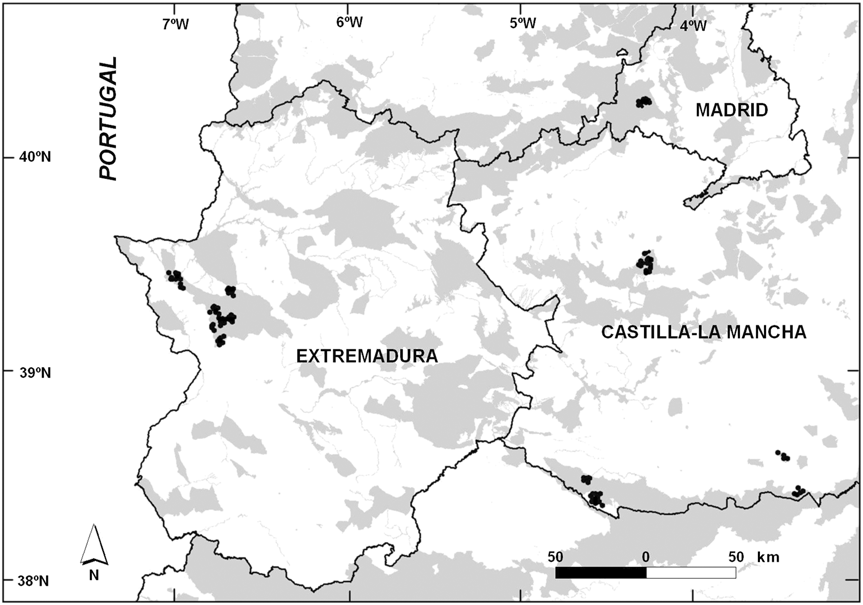
Fig. 1 The study area in Spain, with the location of the 85 studied ponds (black dots). The grey shading indicates Special Protected Areas for birds.
Methods
Black stork surveys
Data were collected during March–September from 2004 to 2007, coinciding with the presence of the black stork in the study area. The ponds were visited monthly to assess the presence of the stork, and only observations of actively feeding individuals were considered. The entire water surface was surveyed using telescopes and binoculars, mostly during 4 hours after sunrise and 2 hours prior to sunset, the most active feeding time of this species (Moreno-Opo et al., Reference Moreno-Opo, Arredondo, Soria, Guil, Higuero and Guzmán2009). Black storks were aged according to plumage and the colouration of unfeathered parts (i.e. beak, legs and eye ring) and classified as juveniles (birds in their first year of life), subadults (birds 2–3 years old) or adults (4 years or older; Cramp, Reference Cramp1998; Ferrero & Pizarro, Reference Ferrero and Pizarro2003).
Data collection
Previous studies have suggested that location, topography, habitat quality, human disturbances, pond productivity, vegetation and food availability may determine the feeding preferences of the black stork (Hancock et al., Reference Hancock, Kushlan and Kahl1992; Cramp, Reference Cramp1998). A total of 17 variables that provide information on these topics were selected (Table 1). Eight of the variables were measured monthly (time-varying variables) and nine, related to the characteristics of each pond (time-invariant variables), were measured once only, where necessary using ArcView v. 3.1 (ESRI, Redlands, USA). For the continuous time-varying variables the mean of all sampling occasions was calculated.
Table 1 The 17 measured variables, ordered by the three groups of variables used in the PCA (see text for details), to evaluate pond selection made by the black stork Ciconia nigra in central and western Spain.
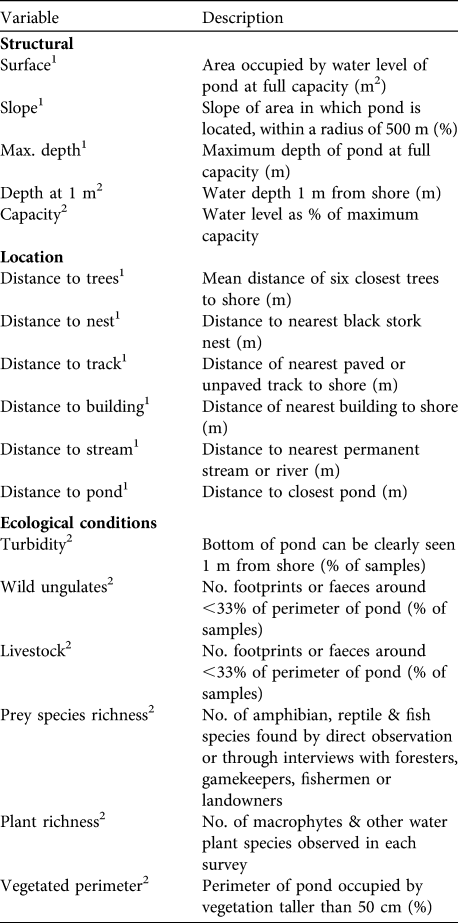
1 Time-invariant variables
2 Time-varying variables
Statistical analyses
The dependent variable was the presence or absence of black storks; the species was considered present at a pond if it was recorded feeding during any visit. We calculated Spearman’s rank coefficients to check for correlation among the 17 predictor variables. Then, because of potential collinearity, and to reduce the number of variables, we performed a principal components analyses (PCA) for each of three groups of variables (structural, location and ecological conditions; Table 1); this grouping facilitates ecological interpretation of the resulting principal components (Crawley, Reference Crawley2007; Williams et al., Reference Williams, Acevedo, Gortázar, Escudero, Labarta, Marco and Villafuerte2007). PCA computes variables that are linear combinations of the original variables and that are uncorrelated with each other. However, the components calculated in each of the three groups can be correlated with components in the other groups. We therefore created a set of competing models in which we checked that components from different groups included in the same model were not correlated, using Spearman’s rank correlation test.
Competing models were fitted using Generalized Linear Mixed Models (GLMM) with binomial errors and a log link function. All models included the random factor Special Protected Area, to correct for any unmeasured variation associated with the pond location. To examine whether the presence of livestock around the ponds can influence presence of black storks we repeated the analysis using only the 44 ponds in areas with livestock.
In all cases models were simplified by removing non-significant factors (α = 5%) after checking, using the likelihood criteria, that the simplification did not significantly change the model. Once we obtained a minimal adequate model for each competing model we chose the most parsimonious using the Akaike Information Criteria (Akaike, Reference Akaike, Petrov and Csaki1973). R v. 2.8.0 (R Development Core Team, 2008) was used for all analyses.
Results
Black storks were detected at 20 ponds (23% of the total) in 34 sightings (1.42% of surveys). Sightings were during the post-breeding (41.2%), migration (35.3%) and breeding (23.5%) periods. The total number of storks observed was 49, with adults (70.7%) more abundant than subadults (19.5%) and juveniles (9.8%). From the PCA we chose the first three components, which explained 78.6, 75.7 and 69.2% of the variation, respectively, in the structural, location and ecological conditions variable groups. Table 2 shows the influence of each variable on the three components in each of the three groups of variables.
Table 2 The eigenvalues of the PCA, for the first three principal components only (see text for details), for each of the three groups of variables (structural, location and ecological conditions; Table 1) for the 85 ponds. The components that were retained in the GLMM analysis were PC1 in the structural group, PC3 in the location group and PC2 in the ecological group (see text for details).
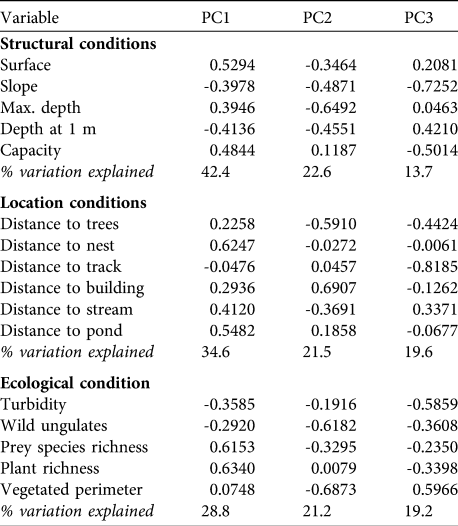
From these nine components (three in each of the three groups of variables), we discarded any that had significant between-group correlations (structural PC2 / location PC2: ρ = -0.37, P < 0.001; location PC2 / ecological PC3: ρ = 0.26, P = 0.018; structural PC3 / ecological PC2: ρ = -0.29, P = 0.008). We built four alternative GLIMM models and selected that with the lowest AIC as the most parsimonious. It included structural PC1 (1.0482 ± SE 0.3315; z = 3.162; P = 0.001), location PC3 (-0.9973 ± SE 0.3303; z = -2.959; P = 0.003) and ecological PC2 (-0.8214 ± SE 0.3498; z = -2.348; P = 0.019).
The results indicated that larger ponds with higher water levels and shallower shores, situated in flat open areas, are positively selected by the black stork. In terms of location, the distance to roads is the most important, with ponds farther from roads used more by the stork. A vegetated perimeter, none or few traces of wild ungulates on the shore, greater prey–species richness and lower turbidity also favour the presence of the species.
The black stork was seen at nine of the 44 ponds in estates with livestock exploitation. Following the same analysis procedure we found that the intensity of use of the ponds by livestock did not significantly influence the presence or absence of the black stork.
Discussion
As direct observation was used to determine the presence of the black stork at ponds it was not possible to determine whether black storks had fed routinely at a particular pond. Nevertheless, the high number (2,380) of our visits over 4 consecutive years increased the chances of detecting the species at any particular pond. The intensity of detection could, however, be improved by using camera trapping.
Feeding habitat selection
The positive relationship between the size of a pond and its selection by feeding black storks may be due to relatively greater heterogeneity and diversity of resources in ponds with a larger water surface area (Oertli et al., Reference Oertli, Auderset, Castella, Juge, Cambin and Lachavanne2002; Kadoya et al., Reference Kadoya, Suda and Washitani2004). Similarly, large ponds hold water for a longer period, even during the summer. This is a key factor in productivity and food availability in wetlands, particularly for secondary consumers (Maheswaran & Rahmani, Reference Maheswaran and Rahmani2002; Taft et al., 2002; Holm & Clausen, Reference Holm and Clausen2006). The negative relationship between deep water around shores and the presence of feeding black storks could be explained by the hunting behaviour of the species (Kahl, Reference Kahl1971). In general, the black stork walks through wetlands trying to locate and harpoon prey in the water, such that the depth of the water cannot exceed the approximate height of their legs (Cramp, Reference Cramp1998). The positive relationship between black stork presence and ponds in flat open areas could be related to the chances of detecting potential predators or other disturbances and the possibilities of successful alert flight (Fernández-Juricic et al., Reference Fernández-Juricic, Jiménez and Lucas2002).
Ponds selected by black stork for feeding are far away from roads frequented by humans. The species is sensitive to human presence and to the effects of certain anthropogenic activities during the summer (Rosenvald & Lôhmus, Reference Rosenvald and Lôhmus2003; Cano et al., Reference Cano, Franco, Pacheco, Reis, Rosa and Fernández2006). It therefore avoids areas where it is more likely that flight would be induced, because this disturbance would reduce its foraging efficiency with respect to time investment and associated stress (Blumstein et al., Reference Blumstein, Fernández-Juricic, Zollner and Garity2005; Young et al., Reference Young, Watt, Nowicki, Alard, Clitherow and Henle2005).
Black storks select well-developed coverage of aquatic vegetation along pond perimeters. This could be related to optimization and opportunities for capturing prey: ponds with an adequate abundance of plants and macrophytes and with good water quality have generally higher productivity values (Bilton et al., Reference Bilton, McAbendroth, Bedford and Ramsay2006; Akasaka et al., Reference Akasaka, Takamura, Mitsuhashi and Kadono2010), and hence may provide a greater relative abundance of macroinvertebrates, herpetofauna and fish. The type and frequency of prey capture varies depending on the area, time and age of the bird (Domínguez et al., Reference Domínguez, González, González, Garzón and Llandres1985; Keller & Profus, Reference Keller, Profus, Meriaux, Schierer, Tombal and Tombal1992; Hampl et al., Reference Hampl, Bures, Baláz, Bobek and Pojer2005). The highest energy efficiency is obtained from medium-sized fish (Ferrero & Pizarro, Reference Ferrero and Pizarro2003; Chevallier et al., Reference Chevallier, Baillon, Robin, Le Maho and Massemin-Challet2008). In both breeding and non-breeding birds, prey availability and feeding habitat quality determine the size of the foraging range and the selection of trophic sources (Keller & Profus, Reference Keller, Profus, Meriaux, Schierer, Tombal and Tombal1992; Jiguet & Villarrubias, Reference Jiguet and Villarrubias2004). Food shortages during the breeding season influence productivity in other stork species (Dallinga & Schoenmakers, Reference Dallinga and Schoenmakers1987; Maheswaran & Rahmani, Reference Maheswaran and Rahmani2002) and therefore could also be a limiting factor in the population dynamics of the black stork.
The black stork also prefers wetland sites with a low occurrence of ungulates, which visit ponds to meet their water requirements. The presence of these wild animals can lower water quality, as well as adversely affect water visibility and food availability near the shore (Putman & Moore, Reference Putman and Moore2002; Herrero et al., Reference Herrero, García-Serrano, Couto, Ortuño and García-González2006), which is the typical feeding site for the black stork. Water turbidity has been shown to negatively affect the probability of detection and capture of prey by storks (Abrahams & Kattenfeld, Reference Abrahams and Kattenfeld1997) and may explain why ponds with low turbidity levels seem to be visited more frequently by feeding individuals.
Conservation implications
In only a few studies on small water bodies has it been possible to assess the occurrence and activity of the species that are part of the biological community (Oertli et al., Reference Oertli, Biggs, Céréghino, Grillas, Joly and Lachavanne2005). We have, however, been able to elucidate the factors that determine the presence of a predator, the black stork, in this type of wetland. The presence of black storks is evidence of the favourable ecological conditions of the wetland network close to their nesting sites (Jiguet & Villarrubias, Reference Jiguet and Villarrubias2004). Their foraging ranges are closely associated with the availability of suitable feeding sites and this is reflected, amongst other factors, in breeding success (Newton, Reference Newton1998). Hence, the scarcity of suitable wetlands constitutes one of the main limiting factors for the species (BirdLife International, 2004; Jiguet & Villarrubias, Reference Jiguet and Villarrubias2004). The negative relationship between the distance of selected ponds to permanent fluvial waters illustrates the need for an adequate interconnected network of wetlands with suitable resources, such as rivers and streams, to meet the requirements of the black stork.
There are two main conservation implications arising from this study. Firstly, it is necessary to maintain a connected networks of ponds with high ecological quality. Secondly, it is important to ensure that ponds meet several ecological, topographic and location conditions (Simon et al., Reference Simon, Snodgrass, Cassey and Sparling2009; Ritcher et al., Reference Ritcher, Crother and Broughton2009). This is particularly important in environments where the fluvial network is limited and fluctuations in water availability are pronounced (González-Gajardo et al., Reference González-Gajardo, Sepúlveda and Schlatter2009).
The management of Mediterranean wetlands has rarely taken into account the importance of ponds and their associated fauna and flora (Ministry of the Environment, 1999) but needs to do so because the number of temporary ponds with good water quality is declining (Gallego-Fernández et al., Reference Gallego-Fernández, García-Mora and García-Novo1999; Zacharias et al., Reference Zacharias, Dimitriou, Dekker and Dorsman2007). In addition, protection of natural or man-made ponds and the promotion of connections between them have been excluded from official conservation strategies, which have disregarded the value of these ponds (Oertli et al., Reference Oertli, Céréghino, Hull and Miracle2009; Pinto-Cruz et al., Reference Pinto-Cruz, Molina, Barbour, Silva and Espírito-Santo2009). It is therefore important to apply conservation actions to a habitat that is considered a priority by official legislation (Ruiz, Reference Ruiz2008).
Management recommendations
To improve the conservation of a wide range of Mediterranean wetland-associated species, including the black stork, it is essential to implement conservation actions such as restoration (Rannap et al., Reference Rannap, Lôhmus and Briggs2009; Lesbarreres et al., Reference Lesbarreres, Fowler, Pagano and Lode2010). The construction or adaptation of ponds could be carried out based on the following criteria: (1) water surface area to be as large as possible; (2) located in flat and open areas; (3) shallow water at the shores (< 30 cm); (4) located as far as possible from human roads or activity; (5) located as close as possible to other ponds; (6) absence of wild ungulates or an increase in the number of ponds in an area to reduce the concentration of ungulates; (7) promotion of the presence of amphibians and encouragement of best practices for increasing their relative abundance (Semlitsch, Reference Semlitsch2000); and (8) promotion of the presence of native fish species. To increase amphibian populations it is recommended that access to the shores by livestock is limited, good water quality maintained, the presence of water in the pond during summer prolonged, the water depth increased, aquatic vegetation encouraged and the concentration of nitrogenous compounds reduced (Galatowitsch et al., Reference Galatowitsch, Whited, Lehtinen, Husveth and Schik2000; Jakob et al., Reference Jakob, Poizat, Veith, Seitz and Crivelli2003; Egan & Paton, Reference Egan and Paton2004; Knutson et al., Reference Knutson, Richardson, Reineke, Gray, Parmelee and Weick2004; Rannap et al., Reference Rannap, Lôhmus and Briggs2009). To foster the settlement of native fish species in man-made ponds implementing introduction protocols is essential to avoid negative ecological effects (García-Jalón & Schmidt, Reference García-Jalón and Schmidt1995; Simôes et al., Reference Simôes, Arruda and Simberloff2009; Uchida & Nioue, Reference Uchida and Nioue2009).
Our recommendations have been communicated to the appropriate authorities, both in Spain and Portugal, and to the managers of the private estates that are of importance for the black stork. Various official plans for the conservation of the species in Spain (Castilla-La Mancha and Castilla y León) and Portugal (the National Action Plan) already incorporate information from the results of this study. The development and implementation of a Natura 2000 network of protected areas in the Mediterranean now takes into account the importance of temporary ponds for the conservation of several priority biodiversity elements.
Acknowledgements
This work was carried out in the framework of the monitoring programme of the LIFE–Nature project 03/NAT/E/0050 Conservation of Spanish imperial eagle, cinereous vulture and black stork, developed by CBD-Habitat Foundation with the collaboration of the Castilla-La Mancha, Extremadura and Madrid regional governments, and the Spanish Ministry of the Environment, Rural and Marine Affairs. It was co-funded by the European Commission and the Spanish Ministry of the Environment, Rural and Marine Affairs. N. El Khadir, J. Oria, M. Panadero, R. Jiménez, S. Pla, J. F. Sánchez, M. Mata, L. Ortega, L. López and L. Bolonio helped in different phases of this study. J. M. Tercero, F. Landaluce, Sir G. Grosvenor, L. Carrascosa, P. Maldonado, L. Urquijo, J. Urquijo, J. L. Ardanza, J. M. Arregui, G. Arregui, A. Rebuelta, J. Finat, R. Finat, R. Garay, A. Álvarez de Toledo, I. Oriol, R. Muguiro, M. Narváez, J. Sánchez, E. Pitarch and A. Rengifo provided the facilities to perform the monitoring. J. A. Donázar, L. M. González, A. Margalida and an anonymous referee provided useful comments.
Biographical sketches
Rubén Moreno-Opo works on the management of projects and conservation strategies for threatened species. He is interested in resolving conflicts between wildlife and socio-economic interests through scientific research and application of technology, social awareness and monitoring. Mariana Fernández-Olalla is investigating the ecological relationships between predator and prey species and the development of demographic models for threatened species. Francisco Guil develops conservation projects for the fauna of the Iberian Peninsula, especially for the limitation of non-natural mortality factors and to strengthen prey species populations and habitat management. Ángel Arredondo, Rafael Higuero, Manuel Martín, Carlos Soria and José Guzmán are field technicians with experience in monitoring and protecting threatened species of birds and mammals in Mediterranean environments.





