Introduction
Amphibian populations worldwide are declining at an alarming rate (McCallum, Reference McCallum2007; Hayes et al., Reference Hayes, Falso, Gallipeau and Stice2010), with 32% of amphibian species threatened; i.e. categorized as Vulnerable, Endangered or Critically Endangered on the IUCN Red List (Stuart et al., Reference Stuart, Chanson, Cox, Young, Rodrigues, Fischman and Waller2004). Several anthropogenic factors contribute to their decline, including habitat loss (Houlahan & Findlay, Reference Houlahan and Findlay2003; Cushman, Reference Cushman2006), pollution (Bridges & Semlitsch, Reference Bridges and Semlitsch2000), disease epidemics and climate change (Kiesecker et al., Reference Kiesecker, Blaustein and Belden2001; Ficetola & Maiorano, Reference Ficetola and Maiorano2016). As for many other taxa (Menzel et al., Reference Menzel, Sparks, Estrella, Koch, Aasa and Ahas2006; Thackeray et al., Reference Thackeray, Sparks, Frederiksen, Burthe, Bacon and Bell2010; Chen et al., Reference Chen, Hill, Ohlemüller, Roy and Thomas2011), a shift to earlier breeding as a result of global warming has been reported for several anuran species (Terhivuo, Reference Terhivuo1988; Reading, Reference Reading1998; Corn, Reference Corn2003; Tryjanovski et al., Reference Tryjanovski, Rybacki and Sparks2003). Shifts are stronger for anurans than those reported for trees, birds and butterflies (Parmesan, Reference Parmesan2007), but the consequences of earlier breeding for population dynamics are still unknown.
Neither phenological shifts (Blaustein et al., Reference Blaustein, Belden, Olson, Green, Root and Kiesecker2001), nor population decline (Blaustein et al., Reference Blaustein, Wake and Sousa1994; Richter et al., Reference Richter, Young, Johnson and Seigel2003; Stuart et al., Reference Stuart, Hoffmann, Chanson, Cox, Berridge, Ramani and Young2008) affect all amphibian populations. The reasons for these variations are still being debated and identifying them requires information on breeding ecology and population trends, which is often difficult to obtain, particularly for rare or declining species (Richter et al., Reference Richter, Young, Johnson and Seigel2003).
In amphibians clutch size (the number of egg masses deposited) is an important life history trait that varies between taxonomic groups, populations, and individuals within the same population (Wells, Reference Wells2007; Cadeddu & Castellano, Reference Cadeddu and Castellano2012). At population-level, variation in clutch size depends on food availability and environmental conditions, mainly temperature (Reading, Reference Reading1998), rainfall (Jensen et al., Reference Jensen, Bayley, Blankenship and Camp2003) and hydroperiod (Semlitsch et al., Reference Semlitsch, Scott, Pechmann, Gibbons, Cody and Smallwood1996). Climatic conditions in spring may affect the length of the breeding period (Jørgensen, Reference Jørgensen1984; Reading & Clarke, Reference Reading and Clarke1995), resulting in variations in clutch sizes (Morrison & Hero, Reference Morrison and Hero2003).
Egg clutch counts have been widely used for monitoring amphibian populations (Crouch & Paton, Reference Crouch and Paton2000; Bernini et al., Reference Bernini, Gentilli, Merli and Razzetti2004; Loman & Andersson, Reference Loman and Andersson2007) and are considered a valid indicator of population trends (Meyer et al., Reference Meyer, Schmidt and Grossenbacher1998; Houlahan et al., Reference Houlahan, Findlay, Schmidt, Meyer and Kuzmin2000). Because the number of egg masses deposited can vary significantly between years, collecting data for > 20 years may be necessary to assess the trend of an amphibian population with sufficient precision (Scherer & Tracey, Reference Scherer and Tracey2011).
In addition to providing descriptions of population dynamics, long-term data are needed to identify changes driven by anthropogenic stressors (Magurran et al., Reference Magurran, Baillie, Buckland, Dick, Elston and Scott2010) and the causes of declining amphibian populations (Stuart et al., Reference Stuart, Chanson, Cox, Young, Rodrigues, Fischman and Waller2004). Long-term data improve ecosystem models and forecasts, supporting effective management actions (Giron-Nava et al., Reference Giron-Nava, James, Johnson, Dannecker, Kolody and Lee2017).
Despite its importance for conservation management, long-term monitoring and collection of basic data on fecundity and population trends are often hampered by low funding and unstable political conditions (Elzinga et al., Reference Elzinga, Salzer, Willoughby and Gibbs2001; Estrada, Reference Estrada2014). Only a few egg clutch time series spanning > 10 years are available, including 22–27 years for Rana temporaria (Switzerland; Meyer et al., Reference Meyer, Schmidt and Grossenbacher1998), 16 years for Lithobates sylvaticus (New England; Raithel et al., Reference Raithel, Paton, Pooler and Golet2011), and 10 years for Rana dalmatina (Italy; Bernini et al., Reference Bernini, Gentilli, Merli and Razzetti2004).
Although Palearctic amphibians are less threatened than tropical species, northern Italy is among the Eurasian areas hosting the highest number of endemic and threatened species: 14 out of 44 (32%) are endemic and 14 are categorized as threatened (4 Endangered and 10 Vulnerable) on the IUCN Red List (Stuart et al., Reference Stuart, Hoffmann, Chanson, Cox, Berridge, Ramani and Young2008; Rondinini et al., Reference Rondinini, Battistoni, Peronace and Teofili2013). The Mediterranean region is considered a hotspot for conservation investment (Myers et al., Reference Myers, Mittermeier, Mittermeier, da Fonseca and Kent2000; Brooks et al., Reference Brooks, Mittermeier, Mittermeier, da Fonseca, Rylands and Konstant2002).
The Italian agile frog Rana latastei is a monotypical, endemic species of northern Italy, the Swiss canton Ticino (Grossenbacher, Reference Grossenbacher and Gasc1997) and the Istria peninsula (Burlin & Dolce, Reference Burlin and Dolce1986), and also occurs locally in lowland hygrophilous woods below 500 m in Slovenia and Croatia (Barbieri & Mazzotti, Reference Barbieri, Mazzoti, Sindaco, Doria, Razzetti and Bernini2006). Females lay their eggs in February–April in single clutches (one per year), which are usually deposited within 50 cm of the water surface of river pools, ponds, springs and canals in wooded areas. Deposition sites are typically rich in aquatic vegetation or provide submerged branches and leaves (Barbieri & Mazzotti, Reference Barbieri, Mazzoti, Sindaco, Doria, Razzetti and Bernini2006). R. latastei is an explosive breeder, with most ovipositions occurring within 2–15 days, eggs hatching 10–15 days after deposition, and tadpoles completing their metamorphosis in c. 3 months (Sindaco et al., Reference Sindaco, Doria, Razzetti and Bernini2005). Less than 100 breeding sites are known (Grossenbacher, Reference Grossenbacher and Gasc1997), most populations are fragmented and genetic diversity decreases from east to west and in peripheral populations (Garner et al., Reference Garner, Angelone and Pearman2003). The species is included in Annexes II and IV of the Habitat Directive of the European Union (EC 43/1992) and categorized as Vulnerable on the IUCN Red List. Reasons for concern are water pollution, increasing urbanization and widespread intensive agriculture, which threaten the residual humid deciduous forests of the Po-Venetian plain, one of the most densely populated areas in Italy (Lassini et al., Reference Lassini, Monzani, Pileri, Pedroli, Van Doorn, De Blust, Paracchini, Wascher and Bunce2007; Sindaco et al., Reference Sindaco, Romano, Andreone, Garner, Schmidt, Corti and Vogrin2009). Additional threats are invasive predatory fishes and crayfish, which have a negative impact on amphibian communities (Ficetola et al., Reference Ficetola, Siesa, De Bernardi and Padoa-Schioppa2012a) and may have caused the extinction of R. latastei from part of its range (Ficetola et al., Reference Ficetola, Siesa, Padoa-Schioppa and De Bernardi2012b).
Considering the Italian agile frog one of the most threatened amphibians in Europe, the Standing Committee of the Bern Convention commissioned an Action Plan for its conservation (Edgar & Bird, Reference Edgar and Bird2006) and the species has been reintroduced in five protected areas of the Lombardy region in northern Italy (Scali et al., Reference Scali, Gentilli, Barbieri, Bernini and Vercesi2001; Bernini & Razzetti, Reference Bernini, Razzetti, Picariello, Odierna, Guarino and Capolongo2002). Despite the increasing interest in its conservation, until now only short-term population surveys have been carried out for this species, with the exception of a 7-year census (Bernini et al., Reference Bernini, Gentilli, Merli and Razzetti2004). The Action Plan has highlighted the need for long-term (> 10 years) studies (Edgar & Bird, Reference Edgar and Bird2006) to account for large annual fluctuations of population size. We monitored egg clutch sizes over a 21-year period (1997–2017) in a small protected area in the Lombardy region with the aim of determining long-term variation in agile frog abundance. Because the area was the subject of a 2-year LIFE Nature Project (LIFE03 NAT/IT/000112) to increase habitat suitability for amphibians and Ardeidae during 2003–2005, we also aimed to assess the Project's long-term impact on R. latastei.
Study area
The study area is located at the Site of Community Interest ‘Riserva di Monticchie’ (SCI IT 209000; 450836 N, 093854 E). The Site covers c. 250 ha on the left bank of the River Po, of which 24.5 ha are hygrophilous forest and 225.5 ha are agricultural land. Residual forested areas consist of alluvial woods with alder Alnus glutinosa, white willow Salix alba and common ash Fraxinus excelsior, and deciduous riparian woods with oak Quercus robur and elms Ulmus spp.
R. latastei has been recorded in the area since the early 1980s (Ferri, Reference Ferri and Zuffi2004). In 2003–2005 the LIFE Nature Project ‘Ardeides and Amphibians: habitat conservation in the Monticchie Natural Reserve’ (LIFE03 NAT/IT/000112) aimed to increase the cover of alluvial forests through selective cutting of allochthonous species, planting of 10,300 indigenous trees and shrubs over a 5 ha area, and restoration of water quality and availability in canals and springs by improving the existing canal network and the circulation of ground water arising from the foot of a fluvial terrace (Fig. 1). Since 2004, canals have been periodically dredged to remove sediments and improve water availability.
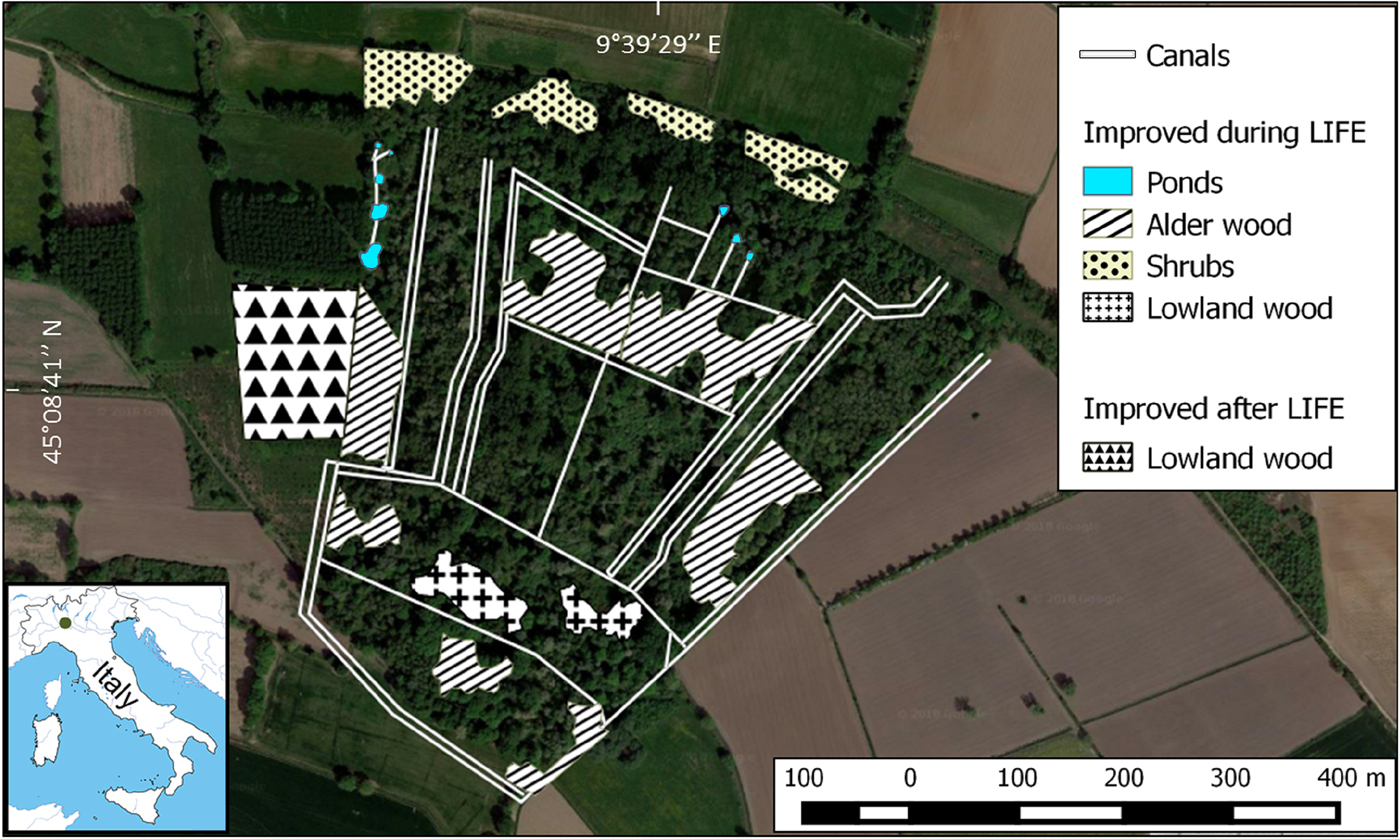
Fig. 1 Aerial view of the study area in 2017. Wooded areas that have been improved during or after the LIFE project (2003–2005) and the canal network are highlighted. The location of the study area in Italy is shown on the inset map.
Methods
From 1997 to 2017 all waterbodies (n = 22) of the site were surveyed 1–3 times per week from early February until c. mid April (depending on rainfall and frog breeding activity) and deposited egg masses were counted. Clutches were detected by walking along the banks of canals (n = 19, total length = 2,340 m, mean length = 120.9 ± SD 69.9 m; mean width = 1.74 ± SD 0.54 m) and small ponds 10–70 cm deep (n = 3, width = 3–10 m). Surveys were usually carried out in the morning, by a main observer and a helper who recorded the main observer's count and any sightings of additional egg masses. Both observers wore polarized sunglasses to reduce the glare of the sun on the water. To help with the identification of newly-laid clutches, all egg masses were photographed and their location marked on a map of the site with landmarks such as trees and stones.
Because the agile frog R. dalmatina, whose egg clutches are similar to those of R. latastei (Bernini et al., Reference Bernini, Gentilli, Merli and Razzetti2004), has never been recorded in the study area despite several surveys (e.g. Ferri, Reference Ferri1988, Reference Ferri and Zuffi2004; Canova & Marchesi, Reference Canova and Marchesi2007) and hydrophone recordings (G. Pavan, unpubl. data), all egg masses could be assigned unequivocally to the target species.
The rate of yearly change in egg mass number was measured using the ΔN method (Houlahan et al., Reference Houlahan, Findlay, Schmidt, Meyer and Kuzmin2000): ΔN = log (N + 1)t − log (N + 1)t−1, where N represents the population size (number of the egg masses) at time t.
The relationship between the start of the breeding season (the ordinal date when the first egg mass was recorded), yearly egg mass counts, and rate of yearly change in egg mass number and climatic and environmental variables was first explored by Spearman's correlation coefficient. The following independent variables were used: (1) year, (2) total precipitation (mm) in February in the years when egg masses were counted, (3) total precipitation during the previous year (rainfall affects adult survival; Berven, Reference Berven1990), (4–6) mean maximum temperature in January (pre-reproductive period), February (start of breeding activity) and February–March (overall reproductive period), (7–9) mean minimum temperature and (10–12) overall mean temperature in the same three periods, (13) air humidity in February, (14) water availability, and (15) mean water depth.
Rainfall (mm), air humidity (%) and mean temperatures (°C) were calculated from daily values obtained from the historical archives of the weather stations of Piacenza and Sant'Angelo Lodigiano (Regional Agency for Environmental Protection), which are c. 15 and 20 km from Monticchie, respectively. Water availability was expressed as the per cent length of canals with water depth ≥ 10 cm; each year, water depth was measured at deposition sites when egg clutches were recorded.
All climatic and environmental variables that were not correlated with any of the dependent variables (P > 0.05) were discarded from subsequent analyses. The influence of the remaining variables on reproductive activity was then analysed using multiple regression. Before the analyses, all variables were tested for normality by Kolmogorov–Smirnov's test. When necessary, data were transformed to achieve normality and homoscedasticity by Box-Cox's method (Reference Box and Cox1964). To avoid multicollinearity, which can inflate the standard errors of the estimates of the model coefficients and produce unreliable results (Hosmer & Lemeshow, Reference Hosmer and Lemeshow1989; Quinn & Keough, Reference Quinn and Keough1993), correlations between the predictors were measured by Pearson's coefficient. Variables to be entered in the model were then selected to omit those clearly representing redundant information; when two variables were correlated, the one to be rejected was chosen according to the strength of its correlation with the dependent variable. The model was then fitted with the predictors in decreasing order, according to the strength of correlation between the dependent variable and each predictor, and following backward elimination of the non-significant predictors (sequential regression method).
Trends through time of climatic variables recorded in February were estimated by regression of each variable on year. Statistical analyses were run in SPSS 21.0 (IBM, Armonk, USA) and PAST 2.17c (Hammer et al., Reference Hammer, Harper and Ryan2001).
Results
The number of egg clutches recorded in a single breeding season ranged from 12 in 2002 to 813 in 2010 (mean = 139.9 ± SD 202.8; Fig. 2). Peaks in the number of eggs deposited by R. latastei occurred 2 years after dredging (Fig. 2). The overall trend in the rate of yearly change in egg mass number was slightly positive over the study period (ΔN = 0.04 ± SD 0.45; Fig. 3).
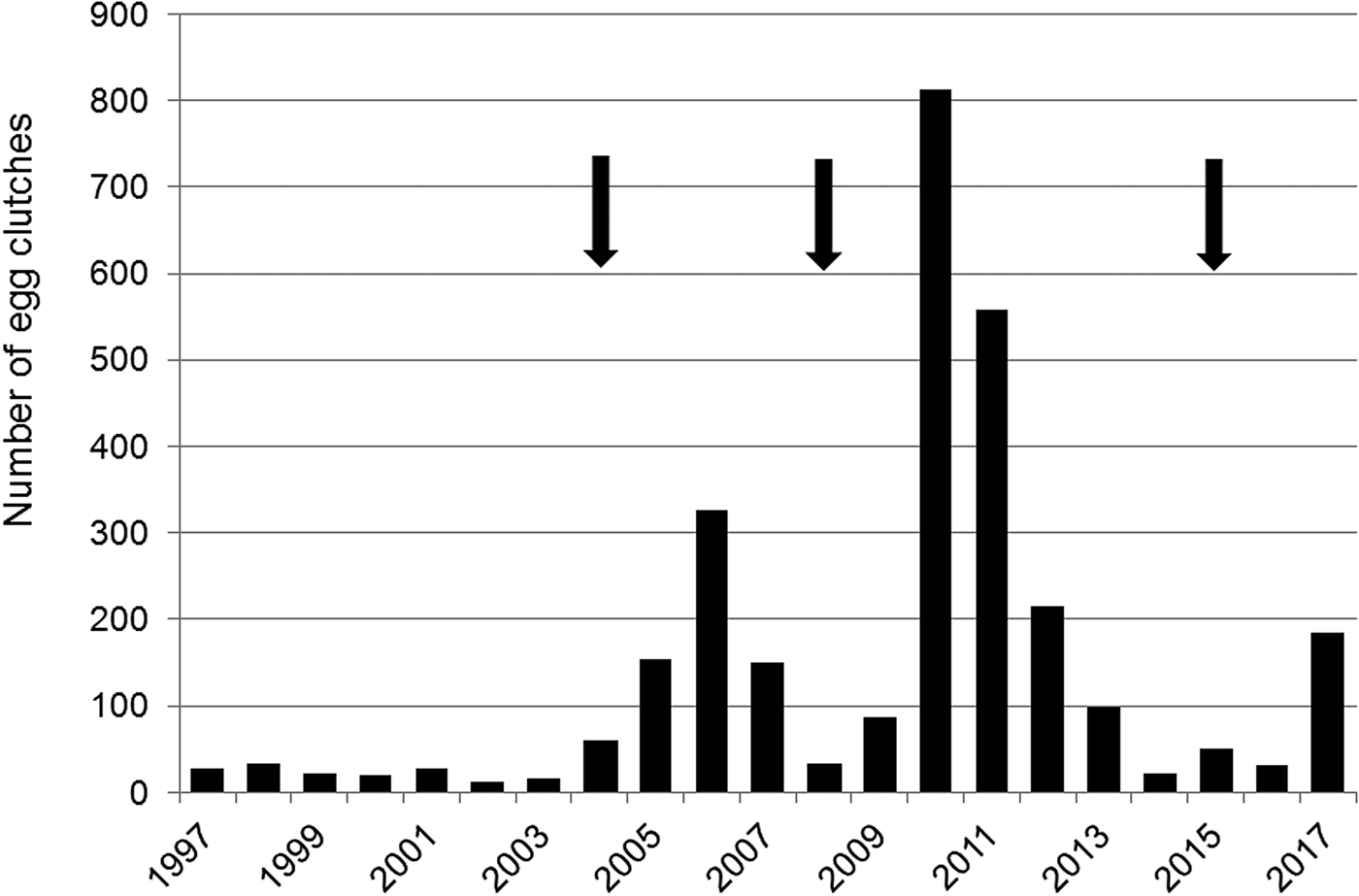
Fig. 2 Yearly variation in the number of egg clutches deposited by Rana latastei at the Site of Community Interest ‘Riserva di Monticchie’ in northern Italy. Arrows indicate the years when the canal network was dredged to increase water availability.
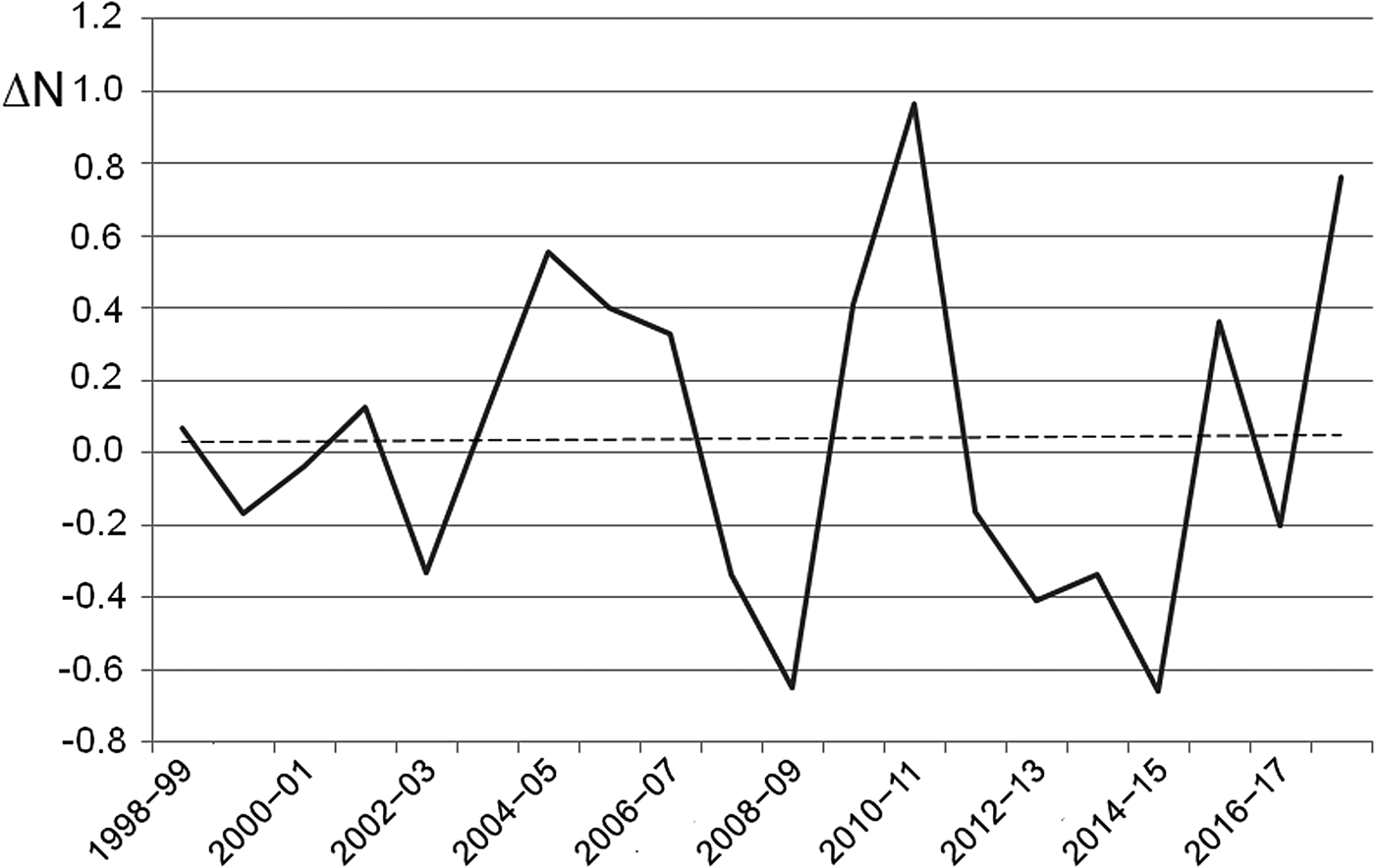
Fig. 3 Rate of yearly change (ΔN) in R. latastei egg clutch numbers at the Site of Community Interest ‘Riserva di Monticchie’ in northern Italy. The dashed line shows the slightly positive overall trend.
Throughout the study period, agile frogs most frequently selected the same group of canals in the centre of the site for egg deposition, with 70% of all egg clutches deposited there. These canals were used in 68–86% of reproductive seasons, whereas 14 out of the 21 monitored canals were used ≤ 4 times, mainly during reproductive peaks.
The number of egg clutches increased with water availability (r = 0.77, P < 0.001) and depth (r = 0.7, P = 0.003), and was inversely related to maximum air temperature in February (r = 0.56, P = 0.007) (Fig. 4). Water availability was the only predictor included in the regression model for the number of egg clutches deposited (b = 0.011 ± SD 0.01, P < 0.0001). When running multiple regressions with only the climatic variables, the final model included the mean maximum temperature in February (b = −0.09 ± SD 0.03, P = 0.008).
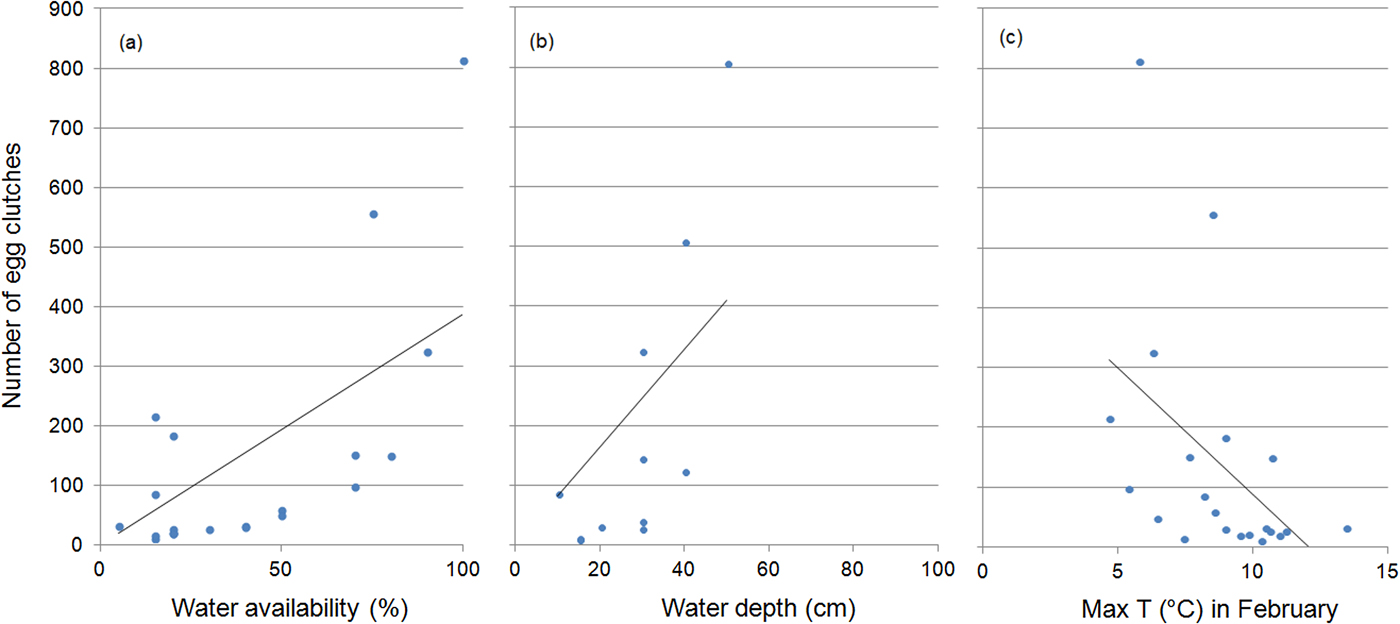
Fig. 4 The effect of (a) water availability, (b) water depth and (c) maximum water temperature on the number of egg clutches deposited by R. latastei in the study area during 1997–2017. Regression lines are shown for each graphic.
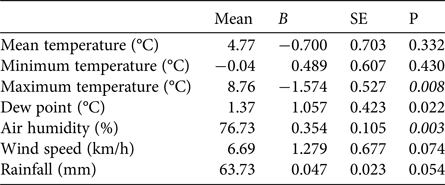
The first egg clutch was detected at a mean of 44.2 ± SD 4.5 days, with the ordinal date of first deposition increasing significantly throughout the study period (b = 2·10−9 ± SD 5.9·10−10, P = 0.003; Fig. 5). The analysis of year-to-year variation in climatic variables showed that the mean daily maximum temperature in February decreased and air humidity increased (Table 1; Fig. 5). Breeding date and maximum temperature in February were inversely related, although not significantly (r = −0.39, P = 0.08).
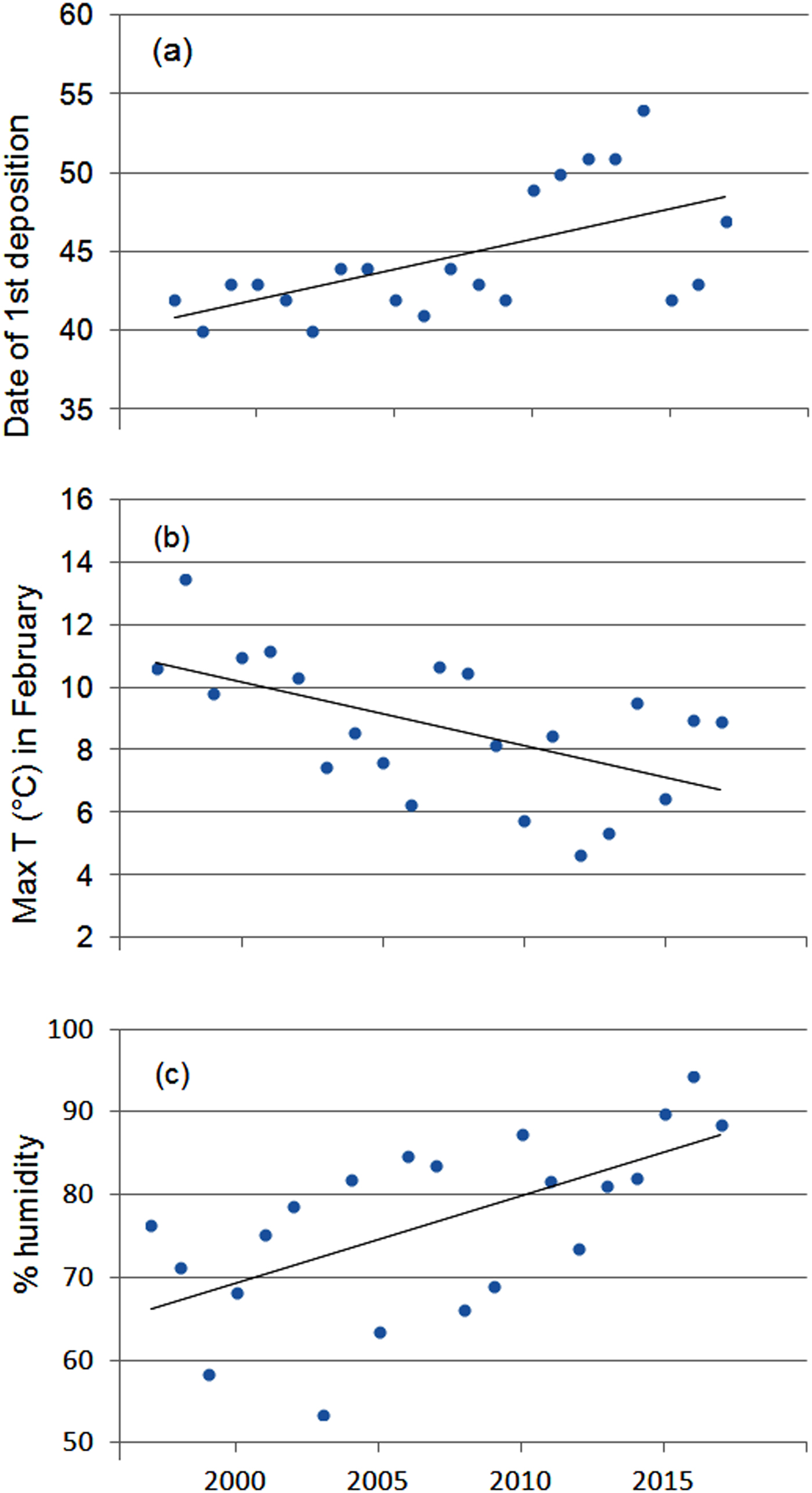
Fig. 5 Variation in (a) date of first deposition, (b) maximum air temperature and (c) humidity in February during 1997–2017. Regression lines are shown for each graphic.
Table 1 Tests for potential trends in climatic variables at Piacenza and Sant'Angelo Lodigiano in February 1997–2017, with mean values and coefficients (b) and their standard errors (SE) from regressions of the variables against year. Significant trends (t-tests P < 0.01) are shown in italics.
Discussion
Egg counts are typically incomplete and represent only a proportion of the actual female population (Campbell Grant et al., Reference Campbell Grant, Jung, Nichols and Hines2005). Although we did not account for detectability, we surveyed each waterbody several times per breeding season, ensuring that our count of egg masses was as complete as possible. Because the same surveys methods were used every year, we expected inter-year comparisons to reflect fluctuations in breeding population size accurately.
Italian agile frogs were consistently found to breed in the monitored area throughout the study period. However, as reported for several other anuran species (Richter et al., Reference Richter, Young, Johnson and Seigel2003; Hartel, Reference Hartel2008; Scherer & Tracey, Reference Scherer and Tracey2011) the number of egg clutches deposited varied significantly between years. Despite this variation in reproductive output, monitoring over 2 decades revealed no clear trend, suggesting an overall stable breeding population.
This result contrasts with the conclusions of the final report of the LIFE Nature Project (Marzatico, Reference Marzatico2005) carried out in 2003–2005, which stated ‘a large increase […] in the amount of eggs deposited’. Although the sudden increase in clutch number recorded during the intervention supported the actions undertaken by project managers, our results reinforce the need for multi-year monitoring to determine the long-term success of species-specific habitat restoration projects (Silva et al., Reference Silva, Houston, Toland, Jones, Eldridge and Thorpe2014). The risk of drawing erroneous conclusions about population trends from the analysis of short-term data has also been highlighted in previous analyses of 25-year long time-series (Meyer et al., Reference Meyer, Schmidt and Grossenbacher1998; Pechmann et al., Reference Pechmann, Scott, Semlitsch, Caldwell, Vitt and Gibbons1991).
Variation in clutch numbers depends on weather conditions such as daily fluctuations in temperature and humidity during the breeding season, rainfall in the previous seasons, habitat changes and demographic patterns (i.e. the survival rate of adults in the pre-reproductive period) (Berven, Reference Berven1990; Reading & Clarke, Reference Reading and Clarke1995; Harper & Semlitsch, Reference Harper and Semlitsch2007; Wells, Reference Wells2007). In the study area, climatic conditions in both the pre-reproductive and breeding periods played a negligible role in determining year-to-year variation in the number of egg clutches deposited. The negative effect of maximum air temperature in February on the number of egg clutches deposited is difficult to explain, although frogs breeding in closed canopy ponds may be less tolerant of high temperatures than those from open areas (Skelly & Freidenburg, Reference Skelly and Freidenburg2000). Because males call underwater (Farronato et al., Reference Farronato, Pesente, Fracasso, Carlotto, Bon and Scarton2000) and water temperature remained constant, variations in air temperature might not affect breeding activity. Rainfall, which has been shown to affect breeding population size in other amphibians (Semlitsch et al., Reference Semlitsch, Scott, Pechmann, Gibbons, Cody and Smallwood1996), did not seem to affect population fluctuations of R. latastei. Similar results have been found for three populations of R. temporaria (Meyer et al., Reference Meyer, Schmidt and Grossenbacher1998).
For anurans that deposit their eggs in standing water (80% of anuran families; Duellman & Trueb, Reference Duellman and Trueb1986), water availability is a major determinant of both distribution (Banks et al., Reference Banks, Beebee and Cooke1994; Rodriguez et al., Reference Rodriguez, Belmontes and Hawkins2005) and reproductive success (Rowe & Dunson, Reference Rowe and Dunson1993, Reference Rowe and Dunson1995). The relationship between clutch size and water availability (per cent length and depth of active canals) recorded in our study confirms the importance of the availability of suitable oviposition sites during the breeding period. The importance of water availability was also indicated by the clutch size peaks following dredging actions, which started in 2004 during the LIFE project and aimed to improve water circulation in the canal network. The 2-year delay between dredging and clutch size peaks may be explained by the time required for the renewal of aquatic vegetation and deposition of leaf litter and wood debris, which agile frogs require for breeding (Barbieri & Bernini, Reference Barbieri and Bernini2004). Another factor may have been that dredging was carried out in November, potentially disturbing hibernating frogs. Canals used regularly for oviposition were located in the inner, less disturbed and more forested part of the study area. These canals showed lower variation in water availability throughout the study period compared to peripheral water bodies and have been used by frogs since the 1980s (Ferri & Agapito Ludovici, Reference Ferri, Agapito Ludovici and Ferri2002), suggesting high site-fidelity. The larger number of canals used during reproductive peaks reflects the higher water availability following dredging and suggests that in dry springs intraspecific competition for oviposition sites may be more intense.
In temperate regions the beginning of breeding activity in explosive-breeding anurans is determined by rising temperatures at the end of winter (Wells, Reference Wells2007; Sofianidou & Kyriakopoulou-Sklavounou, Reference Sofianidou and Kyriakopoulou-Sklavounou1983) and mean air temperature in February can affect the breeding dates of Rana spp. (Terhivuo, Reference Terhivuo1988; Hartel, Reference Hartel2008). In the 20-year period of monitoring, a period long enough to detect shifts in the onset of breeding caused by climate change (Reading, Reference Reading1998; Beebee, Reference Beebee1995; Forchhammer et al., Reference Forchhammer, Post and Stenseth1998), agile frogs tended to breed progressively later. This finding, which contrasts with the global trend, corresponds with the recorded decrease in the daily maximum temperatures in February, although mean temperatures did not vary accordingly. Other studies have also found that some anuran species do not tend to breed earlier or even show a tendency to breed later (Blaustein et al., Reference Blaustein, Belden, Olson, Green, Root and Kiesecker2001; Gibbs & Breisch, Reference Gibbs and Breisch2001), probably as a consequence of local variation in climatic conditions.
Yearly monitoring allowed us to record the actual trend of the local agile frog population, relating large fluctuations in clutch size to water availability and management actions and assessing the long-term success of the LIFE Nature Project. Although pre-metamorphic survival is mainly determined by density dependent factors (Berven, Reference Berven1990), anecdotal evidence suggests that water availability also may be important for agile frog reproductive success during the developmental period. During 1980–2000, most canals occasionally dried up before tadpoles underwent metamorphosis (Ferri, Reference Ferri and Zuffi2004), threatening tadpole survival and their recruitment into the breeding population in subsequent years. Given that rainfall increased only slightly during the study period, drying up was probably caused by either the occlusion of groundwater springs by sediments or water usage for agricultural purposes.
The periodical dredging (every 4 years) of the existing canal network is an important management action to ensure the availability of suitable breeding sites and support the conservation of this agile frog population. The results of our long-term monitoring suggest that in small wooded areas in an agricultural landscape, water availability can be highly variable because of its exploitation for watering surrounding crops, and can become a limiting factor for amphibians. In such cases, we recommend the following management measures: (1) dredging should be carried out regularly to prevent the silting up of canals and ensure a water depth suitable for oviposition and tadpole development (40–60 cm), (2) dredging activities should be carried out at the end of summer, before the species enters hibernation, and (3) long-term monitoring (> 10 years) of the size of the targeted population beginning at the start of a project is required for a full assessment of the success of conservation interventions. Site-fidelity implies that habitat restoration and reintroduction or reinforcing plans need to take into account the historical distribution of the species in the intervention area, and that many years may pass before agile frogs colonize a new area permanently.
Efforts to recreate aquatic and terrestrial habitats to support the long-term viability of agile frog populations have been limited to date (Edgar & Bird, Reference Edgar and Bird2006). Since 2011, the reserve authorities have managed to increase water availability and stabilize water levels at the study site. The first intervention was the construction of an artificial pond, connected to the water table, to which egg clutches can be moved when canals dry up. Authorities are currently planning to construct shallow ponds and marshy areas, covering c. 4 ha, to provide further suitable deposition sites. An additional step may be the creation of a buffer zone to avoid pollution of the inner area during chemical treatment of neighbouring croplands. Our results have also been considered for preparing a Regional Action Plan for the conservation of threatened aquatic species.
Acknowledgements
We thank the administration of Monticchie Natural Reserve and Assessorato Ambiente, Regione Lombardia for funding [GC-32-17042009; GC-99-28122011], permissions and logistic support, P. Mazzoleni for permission to visit his farms, and A. Mascherpa, A. Cremonesi, A. Lucchini, S. Ghidotti and S. Uccellini for their help with fieldwork. G. Pavan recorded and analysed male calls by hydrophone and S.J. Lovejoy provided language editing.
Author contributions
Research conception and clutch size monitoring: LC; data analysis: AB; writing of the manuscript: LC and AB.
Ethical standards
The research was non-intrusive and had the necessary approval and permits from the administrations of Monticchie Natural Reserve and Lombardy Region.
Conflicts of interest
None.










