Introduction
The clouded leopard Neofelis nebulosa is categorized as Vulnerable on the IUCN Red List and is listed on Appendix I of CITES (Reference CITES2014). It is the largest felid in Taiwan and has been categorized as Endangered under the Wildlife Conservation Act since 1989. Lee & Lin (Reference Lee, Lin and Peng1992) suggested that clouded leopards were nearly or already extinct in Taiwan; no direct records of occurrence (e.g. sightings, photographs, carcasses) had been reported since 1983 and pre-1983 records were based on interviews. However, for a species to be categorized as Extinct, IUCN stipulates that exhaustive surveys must be conducted in its range over a time frame appropriate to the taxon's life cycle (IUCN, 2001). No field surveys of clouded leopards have been conducted in Taiwan and information from local people is anecdotal and unsubstantiated (Rabinowitz, Reference Rabinowitz1988). The population status of the species cannot be ascertained without field surveys but if any clouded leopards remain they can only be in very small numbers and in remote areas.
The clouded leopard's prey species have experienced pressure from habitat loss as a result of human encroachment and from hunting, although commercial hunting was banned in 1973 (Lee & Lin, Reference Lee, Lin and Peng1992). However, populations of some prey species, including Formosan macaques Macaca cyclopis and some mammalian herbivores, have reportedly increased, causing conflict on farmlands and in forests. Although these increases could be a result of the hunting ban, reduced predation pressure from the disappearance of clouded leopards cannot be ruled out (Crooks & Soule, Reference Crooks and Soule1999; Chiang et al., Reference Chiang, Pei, Vaughan and Li2012). Disappearance of large apex predators can impose trophic cascades on an ecosystem, with effects on biodiversity (Terborgh et al., Reference Terborgh, Lopez, Nunez, Rao, Shahabuddin and Orihuela2001; Estes et al., Reference Estes, Terborgh, Brashares, Power, Berger and Bond2011). Thus, it is important to assess the population status of the clouded leopard and locate any surviving individuals. Reintroduction of clouded leopards to Taiwan could be considered if extirpation is confirmed.
The success of any conservation action and/or reintroduction of clouded leopards would depend on the availability of suitable prey and habitat. The discovery of clouded leopards would necessitate conservation actions such as habitat improvement and/or restocking the population to increase genetic diversity (Roelke et al., Reference Roelke, Martenson and O'Brien1993). If it were confirmed that no clouded leopards remain, suitable reintroduction sites should be identified and habitat quality and connectivity improved. Our objectives were (1) to investigate whether there are any remaining clouded leopards in Taiwan, (2) to document the altitudinal distribution patterns of major potential prey species, (3) to assess the abundance of prey species, (4) to test whether prey populations are being reduced by anthropogenic hunting, and (5) to identify suitable habitat for restoration and for the reintroduction of clouded leopards.
Study area
Taiwan is an orogenic island of c. 36,000 km2, with a large altitudinal range and a maximum altitude of c. 4,000 m. The vegetation gradient changes from coastal plains and lowland tropical and subtropical rainforest to temperate coniferous forest and alpine grassland at the highest altitudes. The Tawu Mountain area in southern Taiwan (Fig. 1) encompasses two protected areas: Tawu Nature Reserve and Twin Ghost Lake Important Wildlife Area. It contains the largest remaining lowland primary forest and is the location in which, if clouded leopards still occur on the island, they are most likely to be found (Rabinowitz, Reference Rabinowitz1988). There is minimal human disturbance in the area and it is primarily covered by pristine Ficus–Machilus, Machilus–Castanopsis and Quercus forests and Tsuga rainforests along the altitudinal gradient 130–3,100 m over 922 km2.

Fig. 1 Locations of camera-trap sites across Taiwan. The inset shows the location of Taiwan off the coast of China.
Methods
Camera-trap surveys
We conducted camera-trap surveys in national parks, protected areas and fragmented lowlands throughout Taiwan during 1997–2012, mostly using film cameras developed in Taiwan. Film camera-traps were set at c. 2 m height and tilted at 40–60° to face the trail or intersection of trails. In 2009 we started using digital camera-traps from Cuddeback (Wisconsin, USA), Reconyx (Wisconsin, USA) and Bushnell (Kansas, USA) in some of the surveys, and by 2011 film camera-traps had been replaced completely by digital. The digital camera-traps were set at c. 0.5 m height for horizontal detection, to accommodate their sensor design. Such horizontal detection may result in higher variations in the detection range, given the variable terrain. With film camera-traps tilted downwards, the detection area was more consistent for prey comparison across sites. Thus, only film camera-traps were used to assess the prey base, to reduce bias from the variable detection areas at sites with digital cameras.
A more extensive camera-trap survey was conducted in the Tawu Mountain area during 2001–2004 to study prey populations under the most natural conditions, without human disturbance and hunting, using stratified sampling according to altitude (Chiang et al., Reference Chiang, Pei, Vaughan and Li2012). Several camera-trap sites were baited with live chickens and other olfactory, visual or auditory lures to increase the chances of photographing clouded leopards.
Prey assessment
We used the number of photographic events per camera-trap day (O'Brien et al., Reference O'Brien, Kinnaird and Wibisono2003) as an index of relative abundance of prey because of its correlation with population densities of mammalian herbivores (O'Brien et al., Reference O'Brien, Kinnaird and Wibisono2003; Rowcliffe et al., Reference Rowcliffe, Field, Turvey and Carbone2008; Rovero & Marshall, Reference Rovero and Marshall2009). Consecutive photographs of the same species within 1 hour were counted as one event and multiple consecutive pictures of groups of animals (e.g. Formosan macaques) were also counted as single events. The relative abundance index of each prey species was multiplied by the edible percentage of mean adult body weight (g) and summed across all prey species, for use as a prey biomass index. Edible percentage was determined as 65% for prey >25 kg, 80% for prey >4 kg and 90% for prey <4 kg (Emmons, Reference Emmons1987; Pedersen et al., Reference Pedersen, Linnell, Andersen, Andren, Linden and Segerstrom1999; Mills et al., Reference Mills, Broomhall and du Toit2004). However, we estimated that the maximum amount of meat a clouded leopard could obtain from large prey (assuming multiple feeding events) was 50 kg, based on the daily meat consumption and kill rates of other wild felids.
To investigate altitudinal patterns of prey distribution and abundance under natural conditions, without hunting, we compared relative abundance indices for each prey and carnivore species and prey biomass indices between four altitudinal zones in the Tawu Mountain area (150–1,200, 1,200–2,000, 2,000–2,500 and 2,500–3,100 m). These four zones reflect the four major vegetation types in the area. We used Kruskal–Wallis tests to identify if there were significant differences between relative abundance indices and prey biomass indices between the zones. We used Jonckheere–Terpstra tests to identify if there were monotonic patterns of relative abundance indices and prey biomass indices along the altitudinal gradient. We performed one-sided Wilcoxon rank–sum tests to examine the effects of hunting on relative abundance indices and prey biomass indices in hunted and non-hunted areas at altitudes <2,000 m in the Tawu Mountain area. We used R v. 2.15 (R Development Core Team, 2009) to conduct statistical analysis. Jonckheere–Terpstra tests were performed in SPSS v.16 (SPSS, Chicago, USA).
We also conducted a meta-analysis of prey biomass index, including data from other camera-trap studies in Taiwan (Wang, Reference Wang2004, Reference Wang2008; Wu et al., Reference Wu, Wu and Wu2004; Wang & Hsu, Reference Wang and Hsu2005; Wang & Huang, Reference Wang and Huang2005; Hwang & Chian, Reference Hwang and Chian2007), to understand factors influencing prey biomass across the country. The prey biomass index used in this meta-analysis was based on the five largest prey species (macaques and ungulates) because limited data were available for some areas. These five species accounted for almost 99% of the prey biomass index. We hypothesized that prey biomass could be influenced by human activity, distance to central Taiwan and altitude. We used multiple linear regression and the information-theoretic approach (Akaike information criterion, AICc) to compare the full model with three independent variables and models with each variable removed in turn.
In our models the value for human activity was based on three factors: accessibility from roads and villages (0 or 1), hunting pressure (0–3), and history of forest practices or agricultural use (0–5). These three factors were each scaled to 10 and summed to give an overall score for human activity (0–30). Accessibility from roads and villages was assigned a value of 1 if the area was within 5 km of major roads or well-maintained logging roads or within 3 km of aboriginal villages, and a value of 0 if the area was outside these ranges. Hunting pressure was assigned a value of 0 if there was no hunting, 1 if there was occasional hunting, 2 if there was persistent seasonal hunting (i.e. every year during October–April) and 3 if there was persistent hunting all year round. The history of forest practices was determined from literature and field observations and was based on the estimated percentages of forest alteration for agriculture, plantations and clear-cutting.
Habitat assessment
We identified areas of suitable habitat for clouded leopards based on the species’ habitat requirements (Nowell & Jackson, Reference Nowell and Jackson1996; Grassman et al., Reference Grassman, Tewes, Silvy and Kreetiyutanont2005). Natural broadleaf forest, mixed broadleaf–conifer forest (primary or secondary) and old-growth cypress forest were considered potential suitable habitats. We excluded coniferous forest, which usually occurs at >2,500 m in Taiwan, non-forested areas, agricultural land, bamboo forest and clear-cut plantation forest (mostly conifers). We identified potentially suitable habitat using a digitized vegetation map produced by Taiwan Forestry Bureau in 1995. Based on data from other studies (home-range and core-area sizes and distances covered daily; Grassman et al., Reference Grassman, Tewes, Silvy and Kreetiyutanont2005; Austin et al., Reference Austin, Tewes, Grassman and Silvy2007) we included in our analyses forest patches >40 km2 and fragmented patches 4–40 km2 that were within 1 km of contiguous primary habitat. Given that clouded leopards may hunt at forest edges (Grassman et al., Reference Grassman, Tewes, Silvy and Kreetiyutanont2005) we considered a 500 m buffer along the boundaries of areas of suitable habitat. Human encroachment and hunting are common and persistent near villages and along roads, and therefore we considered that suitable habitat must be at least 5 km from villages and 3 km from major roads. If clouded leopards used areas adjacent to villages and major roads they would probably have been observed.
Results
Camera-trap surveys
During 1997–2012 we established 1,249 camera-trap sites over 113,646 camera-trap days in various parts of Taiwan, from the coast to 3,796 m altitude. These included 377 sites (13,354 camera-trap days) in the Tawu Mountain area. The total effort, including 209 sites from other surveys, was 1,458 sites (Fig. 1) and 128,394 camera-trap days. No camera traps recorded clouded leopards.
Prey assessment
Our camera-trap survey yielded a comprehensive record of potential mammalian and avian prey. Twelve mammal species and two phasianid bird species identified as potential prey were photographed (Table 1). We excluded other carnivores as prey because clouded leopards have not been observed killing carnivores, nor have carnivores been found in clouded leopard scats (Griffiths, Reference Griffiths1993; Nowell & Jackson, Reference Nowell and Jackson1996; Grassman et al., Reference Grassman, Tewes, Silvy and Kreetiyutanont2005).
Table 1 Prey species in descending order by weight, with maximum edible weight, mean adult weight, relative abundance index (no. of photographic events per camera-trap day) in four zones of altitude in Tawu Mountain area, Taiwan, during 2001–2004 (Fig. 1), P for Kruskal–Wallis test for altitudinal differences, and P for Jonckheere–Terpstra test for monotonic patterns among the four zones.

1 Minimum of 65% of body weight and 50 kg for large prey and 90% of body weight for small prey
2 Based on our own field data or literature
In the Tawu Mountain area we identified a significant decreasing trend in prey biomass index with increasing altitude (Jonckheere–Terpstra test, P < 0.0001; Table 1). Reeves's muntjacs Muntiacus reevesi had the highest relative abundance index (Table 1) at <2,500 m and accounted for >50% of the prey biomass index at <1,200 m. Formosan macaques were the second most important contributor to the relative abundance index at <2,500 m but contributed less than Formosan serow Capricornis swinhoei and sambar Rusa unicolor to the prey biomass index because they are not as heavy. Although wild pigs Sus scrofa were the second largest prey, because of their low relative abundance index their contribution to the prey biomass index was lower than that of the other four prey species at <2,500 m. Macaques and the four ungulates contributed >99% of the total prey biomass index.
We detected significant decreasing trends in relative abundance index with increasing altitude for macaques, muntjacs, Chinese pangolins Manis pentadactyla, Swinhoe's pheasants Lophura swinhoii, red-bellied tree squirrels Callosciurus erythraeus, and spinous country rats Niviventer coninga (Jonckheere–Terpstra test, all P < 0.003; Table 1). Although there were significant altitudinal differences, no monotonic linear trends were observed for serow and sambar. Only Taiwan white-faced flying squirrels Petaurista alborufus lena, Formosan white-bellied rats Niviventer culturatus and Taiwan partridges Arborophila crudigularis showed increasing relative abundance indices along the altitude gradient (Jonckheere–Terpstra test, all P < 0.003; Table 1); however, these are not important prey species nor do they weigh > 0.5 kg.
At altitudes <2,000 m in the Tawu Mountain area the prey biomass index was significantly lower in hunted than non-hunted areas (P < 0.0001; Table 2) because of the significantly reduced abundance of ungulate and primate species (except wild pigs) in hunted areas (Table 2). In contrast, none of the smaller prey species, which were not targeted by hunters, had significantly lower relative abundance indices in hunted areas than in non-hunted areas (Table 2).
Table 2 Prey species in descending order by weight, relative abundance indices for non-hunted and hunted areas at altitudes <2,000 m in the Tawu Mountain area, Taiwan (Fig. 1), during 2001–2004, and Wilcoxon rank-sum P for hunting impacts.

Based on camera-trap data from across the country, the prey biomass indices of the five largest prey species decreased as human activity increased (F 1,26 = 32.08, P < 0.0001). The full model, which included all three variables (human activity, distance to central Taiwan and mean altitude), best explained countrywide variations in the prey biomass indices of these five species (Table 3) and all three factors were significant. In summary, reduced hunting pressure and human encroachment, lower altitudes and closer proximity to central Taiwan supported higher biomass of clouded leopard prey.
Table 3 Linear-regression models of prey biomass index, based on data for macaques, sambar, Reeves's muntjacs, Formosan serow and wild pigs from camera-trapping studies conducted at 28 sites across Taiwan during 2000–2010.
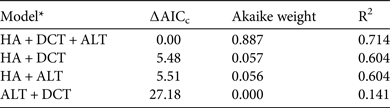
* HA, index of human activity; DCT, distance to central Taiwan; ALT, mean altitude
Habitat assessment
The total area of suitable habitat for clouded leopards, excluding small, isolated fragments, was c. 8,523 km2 (24% of the total land area of Taiwan). After applying the 500 m buffer and excluding areas around roads and villages the area of potential high-quality habitat was reduced to 6,734 km2 (Fig. 2a). The largest continuous block is 2,555 km2, in central/eastern Taiwan and the second largest block, which encompasses the Tawu Mountain area in southern Taiwan, is 2,022 km2 (Fig. 2a). However, a larger portion of the Tawu Mountain area is at altitudes <2,000 m compared to other patches (Fig. 2b). Areas of suitable habitat that are unencroached by humans are fragmented and isolated by roads, agricultural lands and coniferous plantation forests, particularly at altitudes <2,000 m and more so at altitudes <1,500 m (Fig. 2c), where the most abundant prey are found. In summary, the most suitable habitat for the clouded leopard is concentrated in southern and eastern Taiwan.

Fig. 2 (a) Distribution of suitable habitat for the clouded leopard Neofelis nebulosa in Taiwan, excluding areas close to roads and villages, (b) suitable habitat at < 2,000 m altitude, and (c) suitable habitat at < 1,500 m altitude.
Discussion
The mean number of camera-trap days required to record a clouded leopard or Sunda clouded leopard Neofelis diardi in other South-east Asian countries is 113–879, with as few as 8–24 camera-trap sites (Lynam et al., Reference Lynam, Kreetiyutanont and Mather2001; Kawanishi & Sunquist, Reference Kawanishi and Sunquist2004; Rao et al., Reference Rao, Myint, Zaw and Htun2005; Azlan & Lading, Reference Azlan and Lading2006; Azlan & Sharma, Reference Azlan and Lading2006; Johnson et al., Reference Johnson, Vongkhamheng, Hedemark and Saithongdam2006; Cheyne & Macdonald, Reference Cheyne and Macdonald2011). Carbone et al. (Reference Carbone, Christie, Conforti, Coulson, Franklin and Ginsberg2001) suggested that 1,000 camera-trap days was sufficient time to detect tigers Panthera tigris at low population densities (0.4–0.7 per 100 km2). The area of suitable habitat for clouded leopards in Taiwan is c. 8,523 km2. If there were only one tiger in this area the predicted camera-trapping effort to detect its presence would be c. 85,000 trap days. Given our camera-trapping effort of 128,394 camera-trap days without success, it is unlikely that clouded leopards still exist in Taiwan.
Carbone et al. (Reference Carbone, Mace, Roberts and Macdonald1999) suggested that carnivores weighing <21.5 kg feed mostly on prey that is ≤45% of their own weight. Adult Formosan macaques and Reeves's muntjacs have a mean weight of 9.5 kg, which is c. 41–86% of a clouded leopard's body weight (11–23 kg) and is approximately equal to the mean weight of confirmed prey of clouded leopards, based on scat analyses and field observations (Griffiths, Reference Griffiths1993; Grassman et al., Reference Grassman, Tewes, Silvy and Kreetiyutanont2005). Formosan serow, which are similar in weight to clouded leopards, could also be an important prey species as clouded leopards have been observed feeding on goats cached in trees (Hazarika, Reference Hazarika1996). Although clouded leopards are reportedly capable of killing sambar weighing 165 kg (Swinhoe, Reference Swinhoe1862; Kano, Reference Kano1930; Nowell & Jackson, Reference Nowell and Jackson1996), a skull analysis suggested that large prey need to be partially restrained for clouded leopards to deliver a powerful bite at the nape of the neck (Therrien, Reference Therrien2005). Eurasian lynx Lynx lynx regularly prey on reindeer Rangifer tarandus up to 4–8 times their body weight but the reindeer are generally those in poor body condition (Pedersen et al., Reference Pedersen, Linnell, Andersen, Andren, Linden and Segerstrom1999). We speculate that sambar preyed upon by clouded leopards are mostly smaller, weaker or younger individuals. Thus, the importance of sambar in terms of prey biomass index may be inflated. Wild pigs had low relative abundance indices and may be too aggressive to be targeted even by large felids such as leopards (Hayward et al., Reference Hayward, Henschel, O'Brien, Hofmeyr, Balme and Kerley2006).
Potential clouded leopard prey in Taiwan can be divided into two categories by weight (Table 1): ≥ 9 kg (macaques and ungulates) and ≤1 kg (birds and rodents). Chinese pangolin, arboreal flying squirrels and smaller carnivores are excluded because they had low relative abundance indices or there were no confirmed records of them being preyed upon by clouded leopards (Griffiths, Reference Griffiths1993; Nowell & Jackson, Reference Nowell and Jackson1996; Grassman et al., Reference Grassman, Tewes, Silvy and Kreetiyutanont2005). For prey in the ≤1 kg category only Swinhoe's pheasant weighs c. 1 kg; all the others weigh <0.4 kg. Their contributions to the total prey biomass index was, on average, <0.8% of that of the five largest prey species for all altitudes in the Tawu Mountain area. Clouded leopards may not spend much time travelling in rugged and steep terrain to search for prey <0.4 kg as this would not be optimal for maximizing their energy intake (Griffiths, Reference Griffiths1980). Therefore, larger prey species, particularly macaques, muntjacs and serow, would be of greatest importance to the survival of clouded leopards in Taiwan.
However, these major prey species are targeted by hunters. The prey biomass index of hunted areas at altitudes of <2,000 m could be as low as that at the highest altitudes, where there is no hunting and clouded leopards would rarely occur. Therefore, hunting could reduce the prey base to an unfavourable level for clouded leopards. The five largest prey species, plus Chinese pangolins and flying squirrels, were hunted both extensively and intensively in the past for commercial purposes, and hunting was widespread before the Wildlife Conservation Act of 1989 was enforced. Prey depletion would have been a threat to the survival of clouded leopards in Taiwan over the past decades or even centuries (Karanth & Stith, Reference Karanth, Stith, Seidensticker, Jackson and Christie1999).
Three of the larger prey species (muntjacs, macaques and pangolins) were more abundant at lower altitudes. Sambar, serow and wild pig did not show altitudinal differences in abundance. However, as altitude increased, the three largest prey species (i.e. heavier than a clouded leopard) comprised a higher percentage of total available prey (Table 1). This may not be good for clouded leopards because these larger prey may be difficult to catch (Griffiths, Reference Griffiths1980; Carbone et al., Reference Carbone, Mace, Roberts and Macdonald1999). As the prey base showed a decreasing trend with altitude, we suggest that the primary habitat for clouded leopards is at lower altitudes, particularly <2,000 m. However, both clouded leopards and their major prey populations have suffered habitat loss and hunting pressure as a result of timber harvesting and human encroachment. Thus, habitat loss and prey depletion are the two main factors driving the extinction of the clouded leopard in Taiwan.
As a result of increasing awareness of the need for environmental protection, logging of natural forests in Taiwan was halted in 1991. Forests have been undergoing regeneration and succession, becoming suitable habitats for clouded leopards and their prey again. If habitat around areas of human encroachment could be better managed and/or restored to a less fragmented condition, the best-quality habitat for clouded leopard in Taiwan could cover c. 8,500 km2 or more. Such an area could be inhabited by 500–600 clouded leopards, based on population density estimates from Thailand (> 6 per 100 km2; Austin & Tewes, Reference Austin and Tewes1999), or up to 760 (9 per 100 km2, 95% CI 8–17; Wilting et al., Reference Wilting, Fischer, Bakar and Linsenmair2006), based on density estimates for the Sunda clouded leopard in Borneo. However, these are conservative estimates, because leopards would also utilize nearby areas in addition to the best-quality habitat assessed.
Populations of the clouded leopard's prey species are also recovering as a result of the ban on commercial hunting, and thus, given also the recovery and protection of habitat, the reintroduction of clouded leopards in Taiwan may potentially be considered. The Tawu Mountain area, where vegetation coverage and prey levels are suitable and human activities are limited, would be the ideal place for initiating the re-establishment of the clouded leopard in Taiwan. As genetic (Buckley-Beason et al., Reference Buckley-Beason, Johnson, Nash, Stanyon, Menninger and Driscoll2006; Wilting et al., Reference Wilting, Christiansen, Kitchener, Kemp, Ambu and Fickel2011) and morphological research (Kitchener et al., Reference Kitchener, Beaumont and Richardson2006) did not identify clouded leopards in Taiwan as a distinct subspecies, animals from mainland Asia could be utilized for reintroduction. As wildlife conservation is a priority issue there, and considering the ongoing recovery of habitat and prey, Taiwan could become a global refuge for clouded leopards.
Acknowledgements
Primary funding was provided by Taiwan Council of Agriculture, Taiwan Ministry of Interior, Taiwan Ministry of Education, Virginia Polytechnic Institute and State University (USA), National Pingtung University of Science and Technology (Taiwan), and the Research Fellowship Program of the Wildlife Conservation Society (USA). Additional support included personal donations from Chih-Yuan Tzeng, Chien-Nan Liu and Yuan-Hsun Sun. This work would not have been possible without the assistance of many technicians and volunteers in the field. We cherish the memory of Prof. Patrick Scanlon and M. Yen and dedicate this research to them.
Biographical sketches
Po-Jen Chiang has worked on carnivorans and raptors since 1995 and is interested in tropical conservation and camera-trap surveying. He is developing an autonomous long-duration sound recording survey technique. Kurtis Jai-Chyi Pei's interests include wildlife rescue and research on mammals. Michael R. Vaughan has worked on a long-term study of the American black bear in Virginia, USA. Ching-Feng Li studies vegetation ecology and plant–animal interactions. Mei-Ting Chen studies small carnivores and leopard cats in Taiwan. Jian-Nan Liu works on wildlife physiology. Chung-Yi Lin studies sambar in Taiwan. Liang-Kong Lin has worked on the ecology, taxonomy and phylogeny of small mammals in Taiwan. Yu-Ching Lai is a landscape ecologist.







