Introduction
South-east Asia is experiencing greater rates of habitat loss and increases in other drivers of extinction than other tropical regions, yet it remains relatively poorly studied in comparison (Woodruff, Reference Woodruff2010; Giam & Wilcove, Reference Giam and Wilcove2012). There is also a geographical bias in ecological research within South-east Asia: almost 70% of studies published during 1991–2010 were conducted in Malaysia and Indonesia (Giam & Wilcove, Reference Giam and Wilcove2012). Even basic ecological information remains scant for much of mainland South-east Asia, and it is imperative to increase research effort in understudied countries so as to improve knowledge of species distributions and facilitate prioritization of conservation (Giam & Wilcove, Reference Giam and Wilcove2012).
This discrepancy between conservation need and active research is particularly notable in Myanmar. Although the country retains a high percentage of forest cover and is considered to be a globally important hotspot for biodiversity (Webb et al., Reference Webb, Phelps, Friess, Rao and Ziegler2012; Zaw et al., Reference Zaw, Myint, Htun, Po, Latt, Maung and Lynam2014), Myanmar contains the greatest extent of terrestrial areas that have not been surveyed for biodiversity in South-east Asia (Giam & Wilcove, Reference Giam and Wilcove2012). Overexploitation of wildlife for illegal trade, logging, the expansion of commercial agriculture, and dams all threaten Myanmar's biodiversity (Rao et al., Reference Rao, Htun, Platt, Tizard, Poole, Myint and Watson2013). Although security issues associated with political instability have slowed deforestation and preserved biodiversity in many areas (Rao et al., Reference Rao, Htun, Platt, Tizard, Poole, Myint and Watson2013; Donald et al., Reference Donald, Round, Aung, Grindley, Steinmetz, Shwe and Buchanan2015), current rapid social and economic change is likely to spur development projects and exacerbate threats in previously remote areas. There is thus an urgency to assess what remains in such locations and gather data that can inform efforts to protect the country's biodiversity.
Karen State, defined here as all areas under the administration of the Karen National Union, lies in the south-east, bordering Thailand. The State has a complex political history, in which many of its citizens have suffered from violence and oppression, and relatively little development has taken place there. Karen culture has a history of preserving biodiversity through traditional, sustainable practices of agriculture and forestry (KESAN, 2008). The State lies within the Indo–Burma hotspot, a globally important region for conservation (CEPF, 2012). The combination of high biodiversity and communities of local people committed to its protection should make the state a priority area for conservation research; however, previous scientific assessments of biodiversity in Myanmar have not included Karen State for security and political reasons (Zaw et al., Reference Zaw, Myint, Htun, Po, Latt, Maung and Lynam2014). There is burgeoning international interest in the forests in the south under the administration of the Karen National Union, also known as the Tanitharyi Region (Donald et al., Reference Donald, Round, Aung, Grindley, Steinmetz, Shwe and Buchanan2015) but there has been little investment by the scientific and conservation communities in the mountain ranges north of Tanitharyi, where the natural forest cover could harbour species of high conservation concern. We therefore conducted camera-trap surveys in hill forests (800–1,900 m altitude) across northern Karen State to ascertain the presence of mammal species and the potential importance of the area for the conservation of globally threatened species.
Methods
The Karen Wildlife Conservation Initiative conducted six camera-trap surveys during December 2014–July 2015 in four areas of Karen State (Fig. 1; Table 1). All sites had a monsoonal climate, with mean annual precipitation of 1,500–2,000 mm; the rainy season lasts approximately from May to October, the cool portion of the dry season from November to February and the hot portion of the dry season from February to May (KESAN, 2008). Each survey involved 18–22 cameras (total number of camera-trap stations = 115). The spatial distribution of cameras was irregular, with the distance between a given camera and its nearest neighbour ranging from < 10 m to > 3 km (mean 872 ± SD 834 m). Cameras were deployed to maximize the probability of detecting large mammals, particularly Panthera species and their ungulate prey. The majority of cameras were placed on animal trails and ridge tops or near waterholes or similar landscape features deemed by the survey teams to be likely to have a high level of usage by wildlife. Various models of cameras (Trophy Cam HD 1196 and 1194 series, and Nature View Cam HD 1194 series; Bushnell Outdoor Products, Overland Park, USA) and settings (e.g. sensitivity or time delays between captures) were used between surveys. Some surveys were conducted in the wet season and some in the dry season. For all of these reasons, unrelated to ecology or species abundance, there is likely to have been significant variation in detectability between surveys. Therefore, we did not calculate relative abundance indices from capture rates because comparisons using such techniques between areas with varying detectability are not statistically valid (Burton et al., Reference Burton, Neilson, Moreira, Ladle, Steenweg and Fisher2015). Instead, we present the total numbers of records of individual species and the percentage of camera-trap stations at which each species was detected as a basis for prioritizing research and conservation.
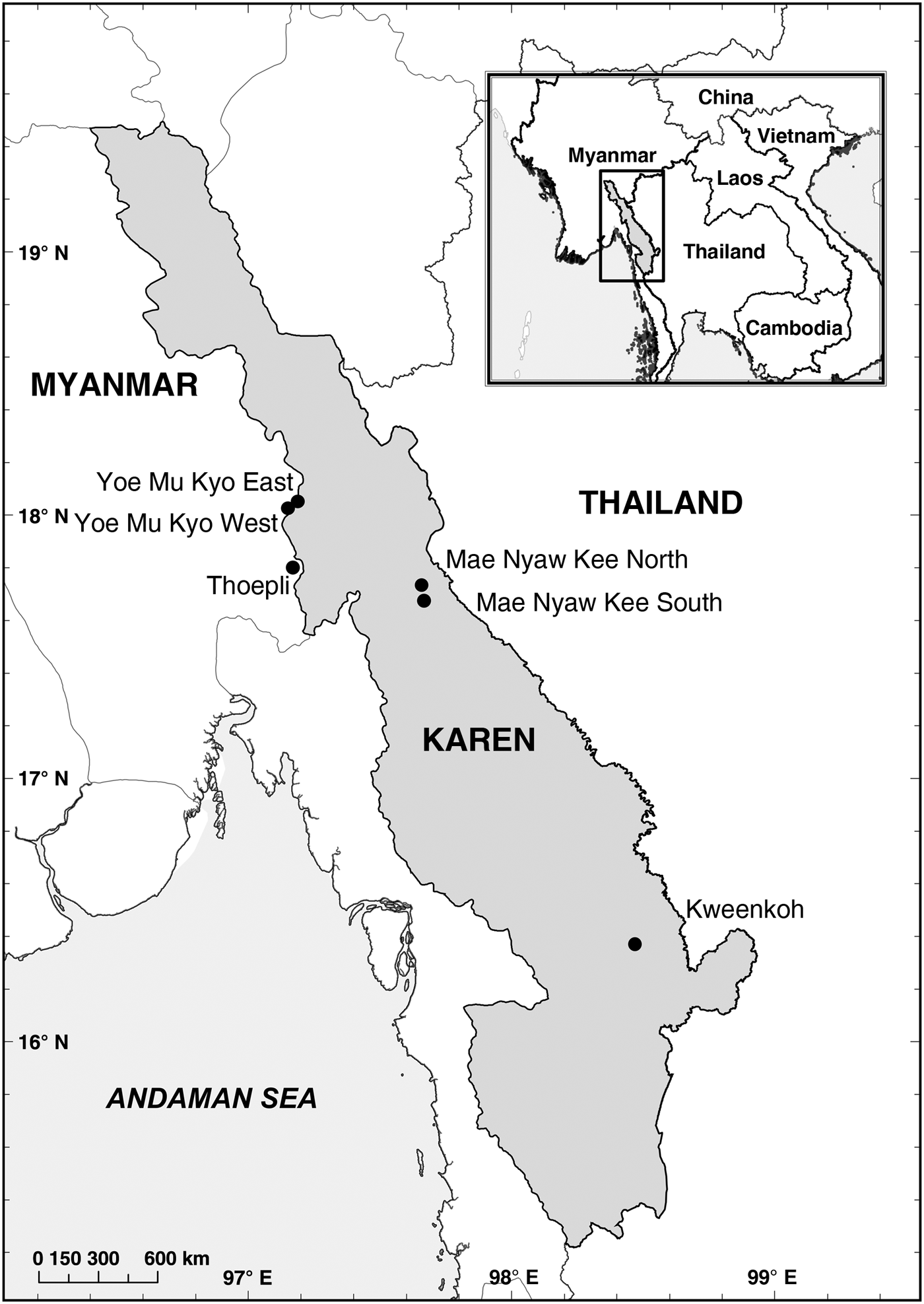
Fig. 1 Locations where camera-trap surveys were conducted in Karen State, Myanmar, during 2014–2015.
Table 1 Details of camera-trap surveys conducted in Karen State, Myanmar, during 2014–2015, with survey site, centroid location, number of successful camera stations (i.e. cameras functioned correctly and yielded usable data), mean number of trap days per station, total number of trap days, mean separation of camera traps, altitude of surveyed area, size of surveyed area, and season.
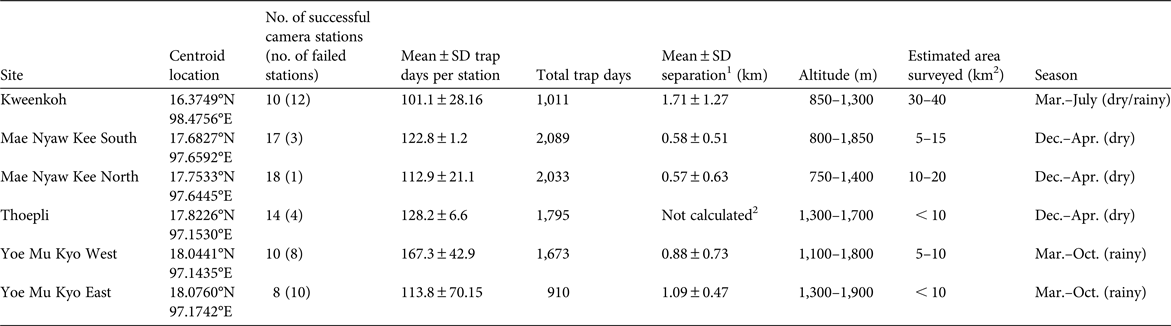
1 The distance between a given camera trap and the closest trap to it.
2 Separation could not be calculated accurately at Thoepli because many traps had corrupted global positioning system data.
Consecutive photographs of the same species at a single station were considered to be separate records if they were taken > 30 minutes apart. This time period was chosen arbitrarily but should be sufficient to ensure that animals travelling together were not recorded as separate records; trial analyses using longer periods had a negligible influence on our results. We excluded bird species and reptiles from the analysis. Murids (sensu lato) were also excluded because they are difficult to identify reliably. Detailed data on tigers Panthera tigris and Asian elephants Elephas maximus were excluded as a precaution, until effective law enforcement measures can be implemented. Our camera traps captured a high number of poor-quality images because of inclement weather, camera angles being displaced by wildlife disturbance, and other factors. Many captures were unidentifiable to species, yet of a size and shape indicating they were mammals. We categorized these at the most specific taxonomic level possible, such as unidentified ungulate, unidentified palm civet or, if completely unidentifiable, unknown. Photographs of linsang Prionodon spp. and langurs Trachypithecus spp. were sent to external experts (see Acknowledgements) for confirmation of identification.
Results
In a total of 9,511 trap-nights we obtained 4,191 records of at least 31 species of mammals (Tables 1 & 2; Supplementary Plates S1–S5). There was a high rate of camera-trap failure, with 38 of 115 deployed cameras malfunctioning and failing to obtain usable images. Of the 31 species detected, four (including the tiger and the Asian elephant) are categorized as Endangered on the IUCN Red List, 10 as Vulnerable, three as Near Threatened, and one as Data Deficient. The most diverse order was Carnivora, with 19 species recorded, and we found evidence of a close to intact carnivore community (Table 2).
Table 2 Mammal species detected by camera-trap surveys in Karen State, Myanmar (Fig. 1), during 2014–2015, with IUCN status and number of records at each site. As a precaution, records of tigers Panthera tigris and elephants Elephas maximus are not included.
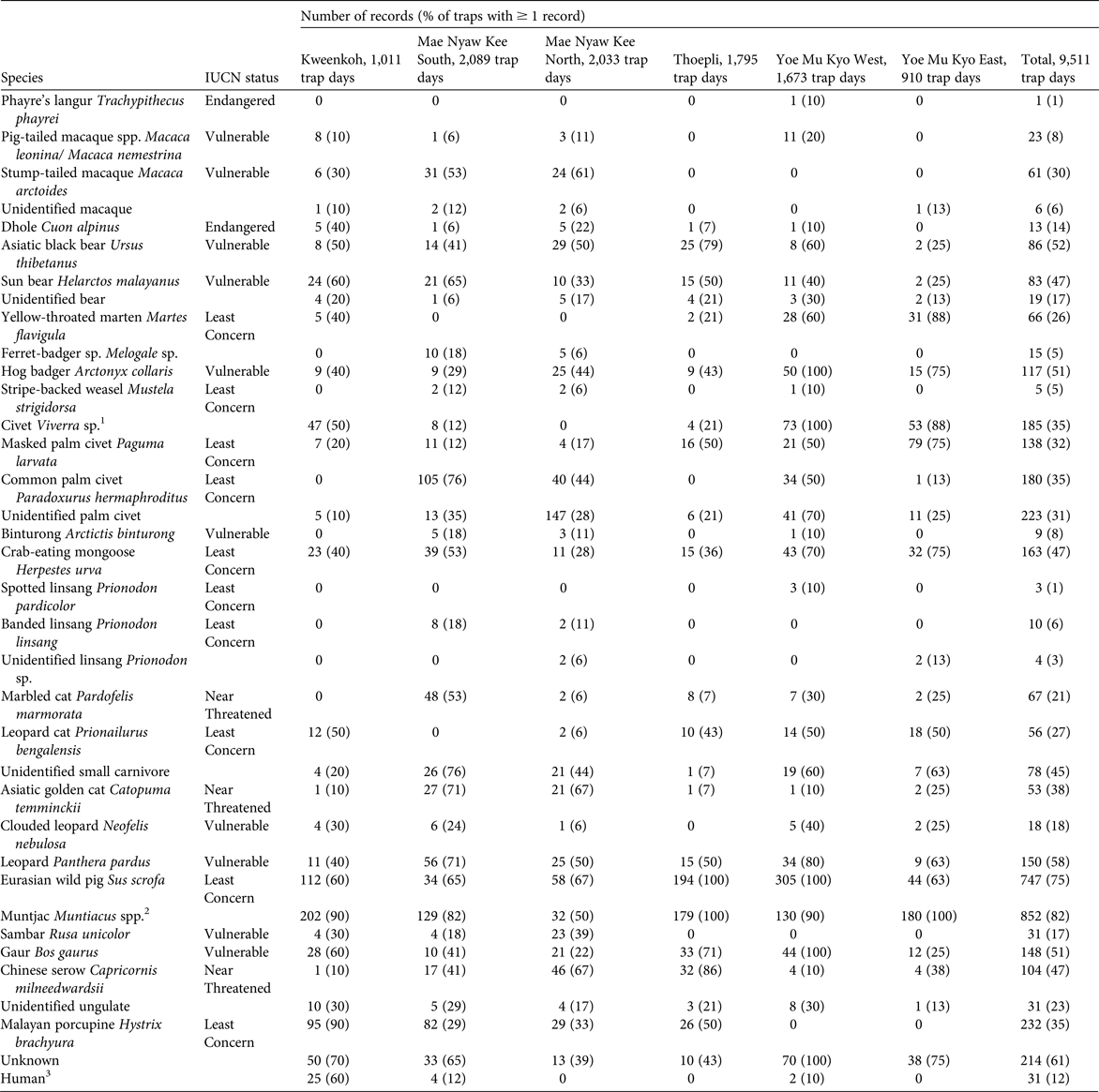
1 The majority of individuals were identified as the large Indian civet Viverra zibetha (Least Concern). Many images were of poor quality and not identifiable to species, so it is possible that some were of large-spotted civets Viverra megaspila (Endangered), although this is unlikely because the species is associated with lower elevations (Zaw et al., Reference Zaw, Htun, Po, Maung, Lynam, Latt and Duckworth2008).
2 Both the red muntjac Muntiacus muntjak (Least Concern) sensu lato (likely to represent the northern red muntjac Muntiacus vaginalis) and Fea's muntjac Muntiacus feae (Data Deficient) were detected, often at the same camera trap. Muntjac images were often of poor quality and many could not be identified to species.
3 Most of the people recorded were confirmed to be hunters.
Discussion
The detection of 17 mammal species categorized as Endangered, Vulnerable or Near Threatened shows that the hill forests of Karen State maintain a significant mammal community. Such species assemblages are either absent or have declined significantly across many areas of South-east Asia (O'Kelly et al., Reference O'Kelly, Evans, Stokes, Clements, Dara and Gately2012; Jacobson et al., Reference Jacobson, Gerngross, Lemeris, Schoonover, Anco and Breitenmoser-Würsten2016). With empty-forest syndrome now prevalent throughout much of mainland South-east Asia, the sympatric presence of so many species represents an increasingly rare opportunity for proactive conservation in the region.
Of particular significance are our detections of tigers, leopards Panthera pardus and Asian elephants. The leopard's distribution and population size have collapsed in South-east Asia and the species is confirmed to occur in < 2.5% of its former range in the region (Jacobson et al., Reference Jacobson, Gerngross, Lemeris, Schoonover, Anco and Breitenmoser-Würsten2016). The population of Indochinese leopard P. p. delacouri is estimated to be 973–2,503 individuals, with only 409–1,051 breeding adults (Rostro-García et al., Reference Rostro-García, Kamler, Ash, Clements, Gibson and Lynam2016). We detected leopards at 58% of all camera-trap stations and across all six survey sites, suggesting that Karen State supports one of the most significant leopard populations remaining in South-east Asia. The status of the tiger in continental South-east Asia is equally dire, with few if any viable source populations outside the central spine of Peninsular Malaysia (Rayan & Mohamad, Reference Rayan and Mohamad2009) and the Western Forest Complex of Thailand (Simcharoen et al., Reference Simcharoen, Pattanavibool, Karanth, Nichols and Kumar2007). Our records extend the current extant tiger range within the Western Forest Complex northwards, and given the ongoing connectivity to source populations in Thailand, the transboundary forests of Karen State and the Western Forest Complex are of global significance for tiger conservation.
We obtained a high number of records of many species across the majority of areas sampled, and a number of significant species (e.g. leopard, gaur Bos gaurus, bear species) were photographed from > 40% of all camera-trap stations. This suggests that abundance may be relatively high, although our methods were not appropriate to estimate rates of occupancy or abundance. For most of the species captured, formal range data in Karen State were unavailable prior to our study, although it was expected that most species would be present in appropriate habitat. However, the records of linsang are significant, with the banded linsang Prionodon linsang encountered further north than previous reliable records had indicated (from Mae Wong National Park, Thailand; Chutipong et al., Reference Chutipong, Tantipisanuh, Ngoprasert, Lynam, Steinmetz and Jenks2014). The detection of the spotted linsang Prionodon pardicolor suggests that Karen State approximates the geographical boundary between the two species.
These surveys are an important first step in providing evidence to support conservation investment in the area. They should be followed by more rigorous monitoring to assess changes in wildlife populations. The Karen Wildlife Conservation Initiative is conducting ongoing camera trapping in a systematic framework to quantify the impact of anthropogenic activity and the effectiveness of conservation action by estimating differences in occupancy across survey areas and over time. Further camera trapping should cover a wider elevational range and variety of microhabitats to increase the probability of detecting species that are known to occur in the area but have not yet been detected, such as the hog deer Axis porcinus, the banteng Bos javanicus, and pangolins Manis spp. Survey teams have observed these species in lowland forest in Karen State.
Although Myanmar retains extensive forest cover (Zaw et al., Reference Zaw, Myint, Htun, Po, Latt, Maung and Lynam2014), the remaining natural forests of northern Karen State are largely restricted to mountain ranges, which run along a longitudinal axis c. 10–20 km wide. That a diverse assemblage of large mammals exists in close proximity to human-dominated landscapes is an encouraging testament to the possibility of human–wildlife coexistence. The stewardship of the Karen people, including socially inherited taboos, which protect threatened species from hunting, is directly responsible for the persistence of biodiversity in the region. The Karen Forestry Department, established in the 1950s, has clearly defined laws, with strong penalties prescribed for hunting of tigers and elephants, and a protected area system based largely on sanctuaries identified under British colonial rule. There are 11 wildlife sanctuaries within the territory of the Karen National Union (Saw, Reference Saw2010) but more comprehensive coverage is necessary to avoid losses in biodiversity and ecosystem services. The Karen Wildlife Conservation Initiative has two trained Wildlife Protection Units operating permanently in the Klermu Thoepli Wildlife Sanctuary but many more units will need to be established throughout the region, for effective wildlife management. This formal protection, together with a respect for cultural traditions and taboos protecting key species and wildlife corridors, has ensured the preservation of the region's threatened mammal species.
However, ongoing political reform in Myanmar and increasing levels of foreign investment in infrastructure projects, including dams on the Salween River, threaten to destroy large tracts of unprotected forest (Donald et al., Reference Donald, Round, Aung, Grindley, Steinmetz, Shwe and Buchanan2015). Meanwhile hunting for the illegal wildlife trade (Nijman & Shepherd, Reference Nijman and Shepherd2015) is a more immediate threat than habitat loss. Of the areas we surveyed, Kweenkoh, in close proximity to the Thai border, appears to be under particular threat, as multiple groups of hunters were captured on camera traps. In the face of rapid economic development, the conservation of biodiversity in Karen State will require a dedicated and urgent international effort. A respectful approach entailing cooperation between all concerned parties is required for success. It is essential that all conservation actions, from the creation of plans to their implementation, are conducted in close collaboration with the people of Karen State, and accurately represent their vision for the landscape.
Acknowledgements
This article was made possible by the hard work and logistical and financial support from all the people involved with the Karen Wildlife Conservation Initiative (KWCI). We thank Saw We Eh Htoo, who helped to coordinate and conduct the surveys, along with other staff members of the Karen Environmental and Social Action Network and the Karen Forestry Department, including, amongst many others, Saw Sunday Poe, Saw Eh Chi and Saw Kyar Lwe Htoo. We also thank Ross McEwing and Paul Sein Twa for their valuable contributions throughout the project. Tun Setha produced the map. We are grateful to the Karen Forestry Department, including Mahn Ba Tun and the forest officers from the districts of Klerlwehtoo, Mutraw and Dooplaya. We thank local district leaders, security guards and local people for helping with transportation as well as hosting and arranging meetings. W. Brockelman, J.W. Duckworth and N. Bhumpakphan assisted with species identification from camera-trap photographs. We are grateful to the Royal Zoological Society of Scotland, Wildlife Asia, WWF network, the European Association of Zoos and Aquaria IUCN South-east Asia Fund and the Balcombe Charitable Trust for financial support for KWCI survey operations.
Author contributions
SSBM and GZLF designed and conducted the study, GZLF analysed the data, and all authors wrote the article.
Biographical sketches
Saw Sha Bwe Moo works on the conservation of biodiversity in Karen State, Myanmar. Graden Froese works on tropical ecology and conservation. Thomas Gray works on large mammal conservation across South-east Asia.





