Article contents
First-principles Study of Electronic and Dielectric Properties of ZrO2 and HfO2
Published online by Cambridge University Press: 11 February 2011
Abstract
Using density-functional theory with ultrasoft pseudopotentials, we previously investigated the structural and electronic properties of the low-pressure (cubic, tetragonal, and monoclinic) phases of ZrO2 and HfO2, in order to elucidate phonon modes, Born effective charge tensors, and especially the lattice dielectric response in these phases. We now extend this previous work by carrying out similar calculations on the two high-pressure orthorhombic phases, and by providing density-of-states and band-gap information on all polymorphs. Our results show that the electronic structures and dielectric responses are strongly phase-dependent. In particular, the monoclinic phases of ZrO2 and HfO2 are found to have a strongly anisotropic dielectric tensor and a rather small orientational average (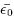 ) compared to the two other low-pressure phases. Our calculations show that
) compared to the two other low-pressure phases. Our calculations show that 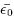 is even smaller in the orthorhombic phases.
is even smaller in the orthorhombic phases.
- Type
- Research Article
- Information
- Copyright
- Copyright © Materials Research Society 2003
References
REFERENCES
- 1
- Cited by


