No CrossRef data available.
Article contents
Axial Concentration Profile of H2 Produced in the CVD of Si3N4.
Published online by Cambridge University Press: 15 February 2011
Abstract
Silicon nitride (Si3N4) has been demonstrated to be an effective high temperature anti-oxidant when deposited in its α-crystalline form. The Materials Technology Laboratory at UTRC has developed a pilot-scale chemical vapor deposition (CVD) reactor capable of depositing α-Si3N4 from ammonia (NH3) and silicon tetrafluoride (SiF4) at 1.8 torr and 1440 C. Coherent anti-Stokes Raman spectroscopy (CARS) has been applied to measure H2 produced in this reactor. Axial concentration measurements have been performed both in the presence and absence of SiF4. Previous CARS measurements demonstrated the importance of surface (Si3N4) catalyzed decomposition of NH3: 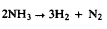
as a competing reaction to: 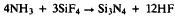
in the CVD reactor under deposition conditions. The observed hydrogen concentration profiles confirm these measurements and allow quantitative comparison between the competing reactions. NH3 decomposition is suppressed 20% by the addition of SiF 4 in a 6:1 (NH3:SiF4) molar ratio. No decomposition is observed in the absence of Si3N4.
- Type
- Research Article
- Information
- Copyright
- Copyright © Materials Research Society 1992


