No CrossRef data available.
Article contents
Activation Energy of Decomposition of Y2bacuo5 Compound in Wet Co2 at Elevated Temperatures
Published online by Cambridge University Press: 28 February 2011
Abstract
Decomposition of the Y2BaCuO5 in C02 was studied using simultaneous thermal analysis (STA) and X-ray diffraction (XRD). The time derivative to the thermo-gravimetric analysis data (DTG) was used to calculate the activation energy. The activation energy of decomposition by CO2 into barium carbonate, copper oxide and yttrium oxide was found to be <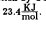
- Type
- Research Article
- Information
- Copyright
- Copyright © Materials Research Society 1990
References
2
Hanagan, M.J. and Simmins, J.J., STAPLOT: A Program for the Analysis of Simultaneous Thermal Analysis Data, Alfred University, Alfred, NY (1989).Google Scholar




