Article contents
Water flow enhancement in amorphous silica nanochannels coated with monolayer graphene
Published online by Cambridge University Press: 10 July 2020
Abstract
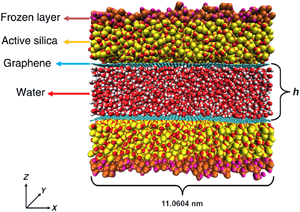
Inspired by the recently reported translucency of monolayer graphene (GE) to wetting, atomistic simulations are employed to evaluate water flow enhancement induced by GE deposited on the inner surfaces of hydrophilic nanochannels. The flow in the coated channels exhibits a slip length of approximately 3.0 nm. Moreover, by contrasting the flow rates in channels with coated walls against flow rates in the corresponding uncoated channels, an “effective” flow enhancement from 3.2 to 3.7 is computed. The probability density function of the water dipole orientation indicates that the flow enhancement is related to a thinner structured water layer at the solid–liquid interface. This study provides quantitative evidence that GE employed as coating reduces substantially hydraulic losses in hydrophilic nanoconfinement.
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society, 2020
References
- 5
- Cited by





