Article contents
Synthesis and characterization of semiconducting sinnerite (Cu6As4S9) thin films
Published online by Cambridge University Press: 12 February 2020
Abstract
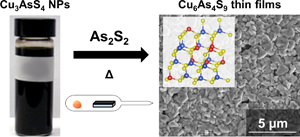
Sinnerite (Cu6As4S9) is a semiconductor computed to have attractive optoelectronic properties, but little attention has been paid to its experimental synthesis and characterization. Here, the authors report the first synthesis of polycrystalline sinnerite thin films. By heating Cu3AsS4 nanoparticles in sealed ampoules with As2S2 powder, a phase transformation to Cu6As4S9 is achieved along with the formation of micron-sized dense grains appropriate for device applications. The films display a bandgap of ~1.2 eV, significant photocurrent generation under simulated AM1.5 illumination, and carrier lifetimes nearing 1 ns, demonstrating the promise of sinnerite for use in photovoltaic applications.
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2020
References
- 4
- Cited by



