Article contents
Preparation and electrical conductivity of graphitic carbon-infused copper alloys
Published online by Cambridge University Press: 29 January 2019
Abstract
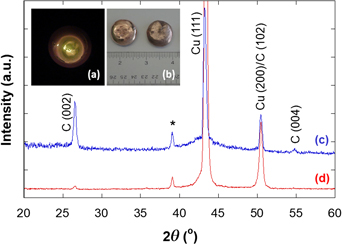
By electron-beam (e-beam) melting, we prepared 0.4 wt% carbon-infused copper (CuCv4), and a copper control without carbon addition (CuCv0). Scanning electron microscopy and helium ion microscopy (HIM) were performed on the as-solidified surface, fracture surface, and ion-polished surface of the CuCv4 sample. The results revealed that graphitic carbon flakes cover the as-solidified surface, and carbon nanoparticles and clusters exist in the fracture and ion-polished surfaces. HIM on the ion-polished surface revealed a unique ripple-shaped feature, which is possibly associated with the infusion of carbon nanoribbons in the copper matrix. The bulk densities were measured to be 8.86 and 8.53 g/cm3, which correspond to relative densities of 98.9% and 96.4% for the CuCv0 and CuCv4 samples, respectively. In addition, apparent electrical conductivities were measured to be 56.9 and 57.5 MS/m, respectively, for the e-beam melted CuCv0 and CuCv4 samples. These values correspond to true electrical conductivities of 100.5% IACS (International Annealed Copper Standard) and 107.4% IACS after correction for the porosity. Our results reveal remarkable promise of using covetic copper for the next generation conductors in energy applications from microelectronic devices to high-power transmission cables.
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2019
References
- 2
- Cited by




