No CrossRef data available.
Article contents
Non-distorted visible light-absorbing thiol-PEGylated gold-coated superparamagnetic iron oxide nanoparticles–porphyrin conjugates and their inhibitory effects against nosocomial pathogens
Published online by Cambridge University Press: 28 October 2019
Abstract
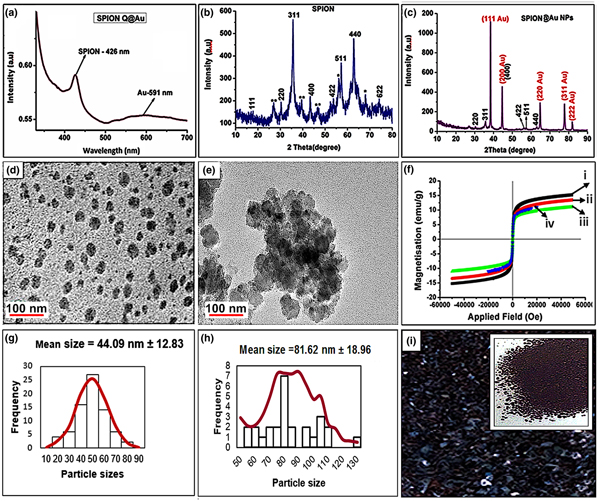
A low-cost synthesis approach was developed for the fabrication of four symmetric meso-substituted water-soluble thiolated polyethylene glycol gold-coated superparamagnetic iron oxide nanoparticles–porphyrin (p-hydroxyphenyl [THPP], 3,5-dimethoxyphenyl [TdMPP], 3-pyridyl[T-3-PyP], and 1-methylpyridinium-3-yl[T3-Py+P4I−]) conjugates to achieve materials with enhanced absorption and therapeutic properties. After evaluation of their antibacterial inhibition characteristics against four nocosomial pathogens (Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, and Enterococcus faecalis), THPP and TdMPP conjugates showed some remarkable minimum inhibitory concentration values of 0.104 and 0.625 mg/mL against E. coli and E. faecalis, respectively, making these materials to be alternative agents for the inhibition of these pathogens in the environmental and clinical fields.
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2019



