Article contents
Interstitial versus substitutional metal insertion in V2O5 as post-lithium ion battery cathode: a comparative GGA/GGA + U study with localized bases
Published online by Cambridge University Press: 22 May 2020
Abstract
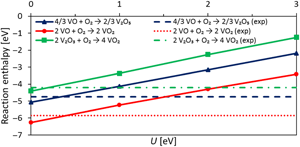
The generalized gradient approximation (GGA) often fails to correctly describe the electronic structure and thermochemistry of transition metal oxides and is commonly improved using an inexpensive correction term with a scaling parameter U. The authors tune U to reproduce experimental vanadium oxide redox energetics with a localized basis and a GGA functional. The value for U is found to be significantly lower than what is generally reported with plane-wave bases, with the uncorrected GGA results being already in reasonable agreement with experiments. This computational set-up is used to calculate interstitial and substitutional insertion energies of main group metals in vanadium pentoxide and interstitial doping is found to be thermodynamically favored.
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society, 2020
References
- 5
- Cited by




