Article contents
Corrosion-resistant nickel thin films by electroless deposition in foam of electrolyte
Published online by Cambridge University Press: 29 January 2019
Abstract
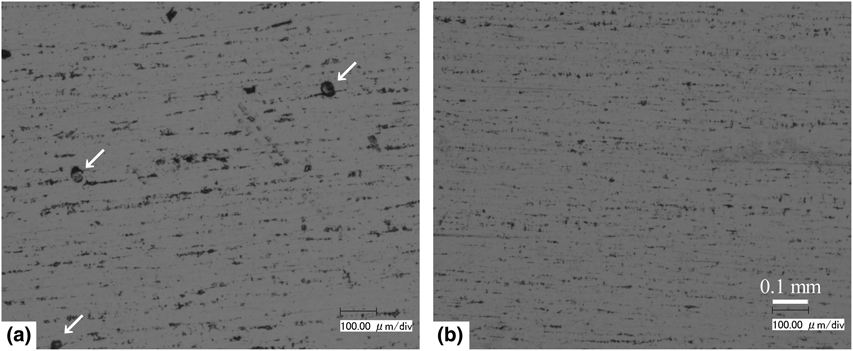
Nickel thin films were prepared by electroless plating in a foam of electrolyte generated by bubbling nitrogen into a hypophosphite-based electroless plating solution added with surfactants of sulfuric acid monododecyl ester sodium salt and ammonium pentadecafluorooctanoate (APFO). Ferroxyl test revealed that the films deposited in foam had substantially higher corrosion resistance than those deposited in liquid. Even with a film thickness of only 1.5 µm, the fraction of corroded area was as small as 0.002% when the film was deposited in the foam. The notable improvement in the corrosion resistance was made possible by adding APFO as the surfactant.
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2019
References
- 3
- Cited by


