No CrossRef data available.
Published online by Cambridge University Press: 20 September 2017
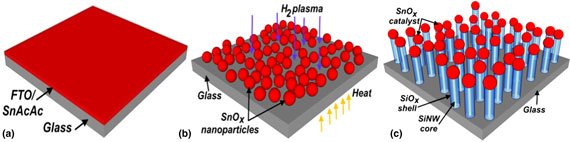
Core–shell silicon–silicon oxide nanowires are synthesized at low temperatures using inorganic and organic compounds of a tin as a catalyst. In situ simultaneous one-dimensional growth of pristine silicon nanowires (SiNWs) using alloy catalyst is reported here. Such a development process generates a high-quality SiNW that is not determined by other atomic species in the plasma. A possible growth model is discussed to understand the synchronized precipitation of a SiNW core and an oxide shell. Nanowires grown here eliminate the additional fabrication steps to deposit anticipated oxide shell that is achieved by precipitation from the same catalyst that precipitates core nanowires.