For understanding the healing and growth of human organs, understanding the effects of mechanical forces on tissue growth is crucial. Bone tissue possesses a certain solid shape with specific elastic properties. However, evidence suggests that tissues, at least at the early stages of growth, often show fluid-like behavior. What seems like a contradiction between the fluid behavior of growing tissue and the final solid shape has been illuminated through research by a group at the Max Planck Institute of Colloids and Interfaces. The work was led by John Dunlop, who is now a professor of biophysics at the Universität Salzburg, and was reported in a recent issue of Science Advances (doi:10.1126/sciadv.aav9394). The researchers’ work elucidates how tissues grow under the influence of the geometry of the surrounding environment and how the aggregate of cells still maintains the shape of the organ. “Cells use surface energy for shaping, in much the same way that complex structures can arise from soap bubbles due to surface energy,” says Peter Fratzl, director at the Max Planck Institute of Colloids and Interfaces and co-author of the work.
The fluid behavior of growing tissue is manifested by the tendency of the tissue to decrease surface area. To explore this behavior, the research group grew tissue on hourglass-shaped curved substrates. A liquid drop of polymers was placed between two surfaces to form a capillary bridge. After it solidified, a substrate with rotational symmetry and constant mean curvature was formed. “Sebastian Ehrig [lead author] made thousands of those substrates with different dimensions to test quantitatively how the curvature of the substrate affects tissue growth; it was years’ worth of effort that culminated in the reported result,” Dunlop says. The tissue grows on the substrate in a way that emulates fluid behavior, similar to the way that water rises in a capillary tube.
The researchers discovered that the cell’s ability to contract creates the necessary surface tension that is responsible for the fluid behavior and subsequently the growth of the tissue. They determined that there is more tissue formed on the substrate when the contractility of the cells is enhanced. “This suggests that mechanical signal transduction between cells and their physical environment, along with the continuous reorganization of cells and matrix, is a key principle for tissue formation,” says Sebastian Ehrig, first author and now a postdoctoral researcher at the Max-Delbrück Center for Molecular Medicine in Berlin.
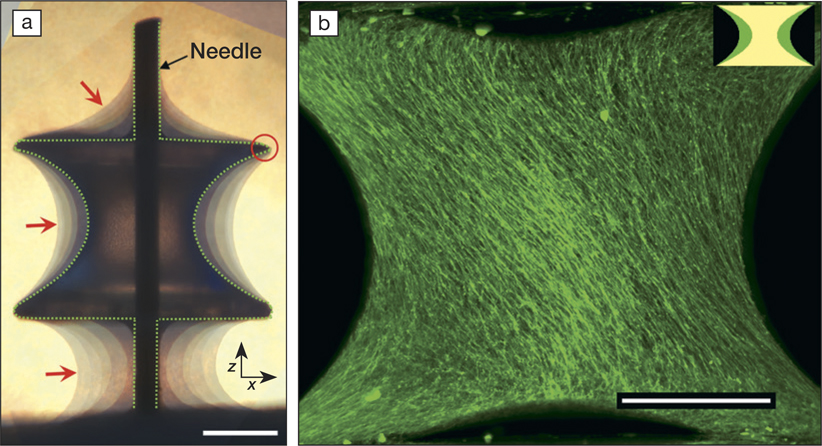
(a) Combination of images of tissues grown on the substrate (capillary bridge or CB) after 4, 7, 21, 32, 39, and 47 days taken by a phase-contrast microscope. The tissue is pinned at the edges of the CB (red circle) and shows a moving contact line in between the substrate and the Teflon holder (holding the substrate through a needle), reminiscent of a liquid. Dashed line indicates the CB surface, and red arrows point toward the tissue–medium interface. Scale bar, 500 µm. (b) Spontaneous emergence of a chiral structure: Maximum projection of tissue grown on a CB with an initial volume of 1.1 µl imaged after 32 days of tissue culture. Tissues were visualized using 3D light-sheet fluorescence microscopy. Scale bar, 500 µm. Credit: Science Advances.
“Cells apply traction forces to each other, and the authors show that the same physical principles that predict the shaping of water/air interfaces can be used to predict the shaping of newly grown cellular systems,” says Viola Vogel, professor and chair of the Department of Health Science and Technology at ETH Zürich in Switzerland.
The group also observed robust behavior of cells self-organizing into left-handed chiral structures. The fibers consistently formed a 60° spiral around the curved surface of the substrates. The researchers’ experiments and theoretical analysis suggest that the chiral behavior cannot be entirely mechanical in origin. Such observations are important in understanding how this preferential direction of growth determines the mechanical properties of tissue.
The research not only taps into basic research to understand cell behavior at a mechanical/physical level and how this affects aggregate behavior and subsequently the tissue shape, but also provides new understanding that could help in the development of future tissue-engineering applications. “Factors shaping embryos during development as well as regulating organ shapes are still not well understood. While much emphasis has been placed on understanding the role of biochemical gradients of signaling molecules, that is, morphogens, as the primary drivers shaping of tissues and ultimately the specialization of cell types within tissues, developmental biology [has] paid little attention to the role of physical forces,” Vogel says. She adds that this quantitative study that analyzed the growth of microtissues in engineered objects “points to the role of mechanical forces and thus surface tension in shaping artificially grown microtissues.”
“What excites me about this work is that we use tools from materials science, differential geometry, and physics to solve problems in biology. We could study how growing tissue behaves like liquids by using physical laws, and consequently our insights could help in future scaffold design,” Dunlop says.


