Researchers have made an all-perovskite tandem solar cell with a record high power-conversion efficiency of 21%.
Tandem solar cells are more efficient than a single cell because each device in the stack can be tailored to absorb a different part of the light spectrum. Tandem perovskite/silicon cells are closer to market, but all-perovskite tandem cells would be easier and less costly.
Making an all-perovskite tandem cell that is efficient has been a challenge. The bottom device in a tandem cell is prepared with a low-bandgap material to absorb all of the infrared photons passing through the top device. Despite many efforts, researchers have had difficulty making high-quality low-bandgap perovskite absorber layers.
Yanfa Yan, at The Uni-versity of Toledo, and his colleagues made a high-quality layer by introducing 2.5% chlorine into a mixed tin–lead perov- skite. This increased grain size and crystallinity of the layer and reduced electronic disorder, which quashed the charge-carrier recombinations that produce heat and boost efficiency of the tandem cell. The cell reported in Nature Energy (doi:10.1038/s41560-018-0278-x) retains 85% of this efficiency after 80 hours.
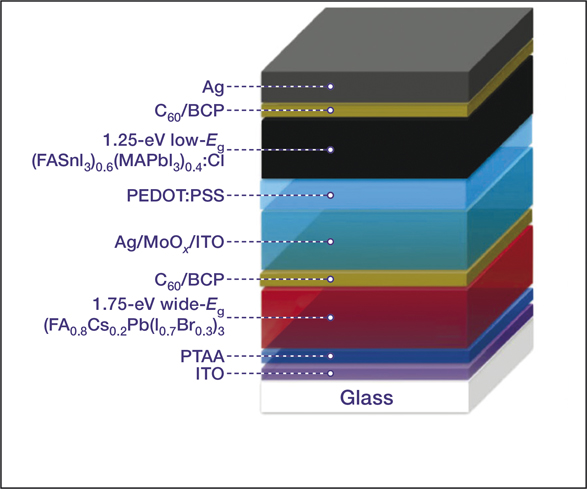
A schematic diagram of the 2T tandem cell structure used in the study. Credit: Nature Energy.
The presence of lead in state-of-the-art perovskite solar cells could hold back their commercialization. Lead-free alternatives based on tin compounds have shown promise, but they typically suffer from low efficiency and stability.
Brown University’s Yuanyuan Zhou and Nitin Padture and their colleagues have made a “surprising discovery” that provides a solution. Simply adding germanium to the lead-free perovskite cesium tin iodide (CsSnI3), which degrades easily, makes it air-tolerant, they found. Devices made with the new CsSn0.5Ge0.5I3 perovskite show an efficiency of 7.11% and remain highly stable after 500 hours of operation under 1-sun illumination. The key to this behavior is the extremely high oxidation activity of Ge, which forms an ultrathin, uniform oxide layer on the surface, “which fully encapsulates and passivates the perovskite surfaces,” the researchers write in their Nature Communications paper (doi:10.1038/s41467-018-07951-y).
Metal-halide perovskite solar cells degrade when exposed to oxygen and moisture. Encapsulating the devices makes them more stable and long-lasting. But it does not solve one issue that crops up during regular device operation. Light, electric field, and thermal stress can all make lead and iodide ions more reactive, generating lead and iodine defects (Pb0 and I0) that serve as recombination centers for charge carriers and bring down device efficiency and lifetime.
Researchers at Peking University in Beijing have invented a novel technique for combating these defects. They added the rare-earth europium ion pair Eu3+-Eu2+ to lead-iodide perovskites. The redox pair shuttled electrons in a cyclical fashion from the defects, oxidizing Pb0 and reducing I0 to recover lead and iodine ions, the team reported in Science (doi: 10.1126/science.aau5701). Devices with this redox shuttle have a power efficiency of 21.52%, and they retained more than 90% of this efficiency under 1-sun continuous illumination or heating at 85°C for 1500 hours.
While lead-halide perovskites have revolutionized photovoltaics, they have also shown promise for lasers, light-emitting diodes, and transistors. Now, researchers show that perovskite nanocrystals are also highly effective catalysts for organic synthesis.
Reactions that form carbon–carbon bonds are the basis of synthesizing drugs, plastics, and chemicals. But the reaction procedures are complicated and require expensive noble metal catalysts. A team led by Yong Yan at San Diego State University found that colloids of methylammonium lead tribromide and cesium lead tribromide are 1000 times as effective as iridium- and ruthenium-based catalysts for catalyzing the α-alkylation of aldehydes, a valuable and widely used chemical reaction. The perovskites cost approximately 100 times less. For the simple one-pot reaction, the researchers mixed organic starting materials into a suspension of the perovskite nanocrystals. Blue-light illumination triggers reactions that generate several products. By tweaking the reaction condition, the researchers can selectively catalyze other important chemical reactions, the researchers report in the Journal of the American Chemical Society (doi:10.1021/jacs.8b08720).


