Following fullerenes, carbon nanotubes, graphene, and graphdiyne, a new carbon molecule—cyclo[18]carbon—has recently joined the carbon allotrope family. This new allotrope consists of 18 sp-hybridized carbon atoms interconnected in a ring form. Due to their high reactivity, purely sp-hybridized cyclo-carbons had never been isolated before, leading to past controversy over their existence and structures. A joint research team led by Leo Gross of IBM Research–Zürich, as well as Przemyslaw Gawel and Harry L. Anderson of the University of Oxford, has settled the dispute. They successfully synthesized and revealed the molecular structure of cyclo[18]carbon. This work was published recently in Science (doi:10.1126/science.aay1914).
The collaboration was initiated three years ago when the Anderson team approached Gross about making cyclo-carbons by leveraging IBM’s expertise for atom manipulation. “The route that was successful in the end seemed like a long shot to me, and I was happily surprised that it worked,” Gross says.
The researchers adopted a voltage-pulse decarbonylation strategy to prepare cyclo[18]carbon. They first synthesized cyclocarbon oxide C24O6 (a precursor) and sublimed it onto a Cu(111) surface partially coated with NaCl bilayers. The substrate was kept at 10 K to stabilize the oxide and loaded into a combined scanning tunneling microscope/atomic force microscope (STM/AFM) instrument. Owing to the atomic resolution of the STM/AFM, the researchers were able to trigger chemical reactions with one precursor molecule at a time by applying 3 V voltage between the STM probe and the precursor molecule for a few seconds. This process eliminated the CO moieties of C24O6 and successfully synthesized cyclo[18]carbon after detaching all six CO moieties. By analyzing the contrast of the AFM images, the researchers concluded that cyclo[18]carbon consists of alternating triple and single carbon–carbon bonds, in contrast to a ring merely composed of double bonds. The synthesized carbon rings have not been separated from the copper substrate due to the high reactivity of cyclocarbons. “It cannot be stable at room temperature in the presence of potential reaction partners,” Gross says.
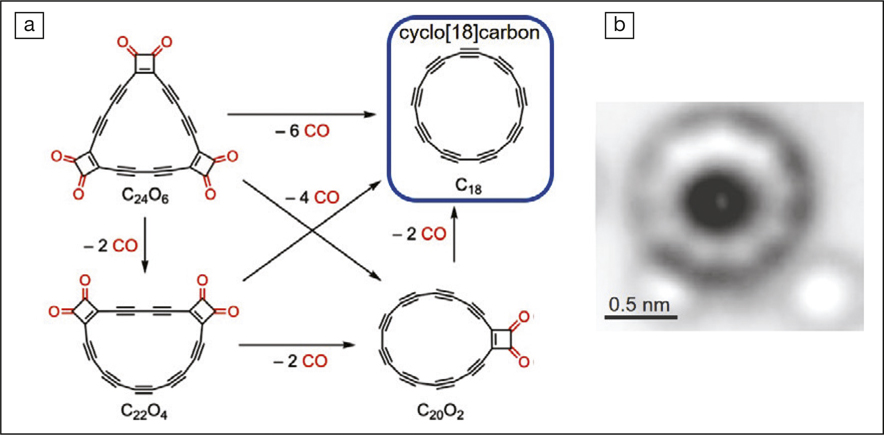
(a) The voltage-pulse decarbonylation process converted cyclocarbon oxides (C24O6, C22O4, and C20O2) to cyclo[18]carbon. (b) Atomic force microscope image of cyclo[18]carbon. The two bright lopes at the lower part are individual CO molecules. Credit: Science.
Changshui Huang of the Chinese Academy of Sciences, whose research group studies graphene and graphdiyne, says this work offers a powerful tool to address the challenge of high reactivity of cyclocarbons. “Low-temperature atom manipulation is a viable strategy to study active molecules. It could open up new opportunities to create and characterize transient and elusive cyclocarbon structures,” Huang says. He was not involved in this study.
The researchers plan to continue collaborations associated with the new carbon allotrope. For example, they have observed that at suitable positions and given sufficient energy, multiple carbon rings can fuse on the NaCl surfaces. Gross says, “With this approach, we hope to create other carbon-rich materials and study them. Possibly we could use these networks as electronic devices, for example, for neuromorphic computing.”




