Noncovalent interactions between soft materials in solution are an essential component of processes such as biodetection and catalysis, and for the development of technologies including microfabricated devices. Despite this, imaging surface interactions remains highly challenging. One recently developed strategy has used the distribution of fluorescent molecular probes on a surface to build a picture of relative changes in chemical topography. In the November 1, 2011 issue of Nature Communications (DOI: 10.1038/ncomms1530), R. Walder and colleagues from the University of Colorado have taken this approach a step further to produce high-resolution maps of the noncovalent chemistry of soft surfaces, using data from the whole surface trajectory of absorbed molecules.
This work uses self-assembled monolayers of trimethylsilane on fused silica as substrates, as these hydrophobic surfaces can be easily micropatterned with hydrophilic regions by exposure to UV light through a suitable mask. Fatty acid molecules that were labeled with the boron-dipyrromethene (BODIPY) fluorophore were chosen as suitable probes, and were applied to the surface in a dilute solution. Each absorbed molecule could then be distinguished using total internal reflection fluorescence microscopy. By taking a series of these snapshots over a period of several minutes, the individual trajectories of the probe molecules over the surface could be tracked. A high throughput analysis of these time-resolved images then allowed real dynamic parameters such as absorption rate, diffusion coefficient, and surface occupancy at each surface pixel to be extracted, and maps of the variation of each parameter to be produced (see Figure).
Each of these maps was able to clearly resolve grids of 10 μm hydrophilic squares where affinity with the fatty acid is much lower than on hydrophobic areas. Further, calibration measurements on a series of homogeneous surfaces with different hydrophobicity demonstrated that single-molecule interactions can be used to probe local hydrophobicity quantitatively on an absolute scale.
The team dubs this new imaging technique “mapping using accumulated probe trajectories” or MAPT and, like other molecular probe methods, its resolution is not diffraction limited. While 125-nm features were resolved in this demonstration, its limits are closely tied to the specific interaction between the probe molecule and the surface. Through appropriate choice of probe molecule, it should be possible to extend the technique beyond hydrophobic interactions to mapping variation in surface reaction rates or conformational changes in surface proteins.
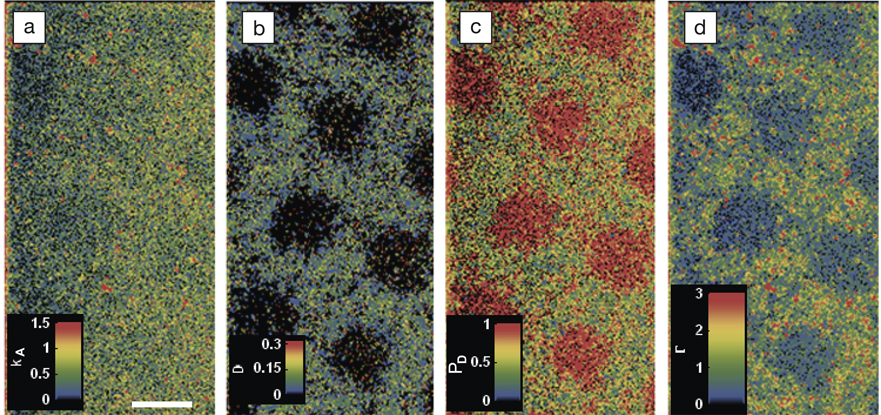
Mapping using accumulated probe trajectory (MAPT) images of (a) adsorption rate, kA (1013 μm−2s−1M−1) (scale bar is 10 μm); (b) average diffusion coefficient, D (μm2/s); (c) desorption probability per 400 ms, PD; and (d) surface coverage/occupancy, Г (1013 μm−2s−1M−1) on a photo-patterned trimethylsilane surface. Reproduced with permission from Nat. Commun. 2:515, DOI: 10.1038/ncomms1530. © 2011 Macmillan Publishers Ltd.


