Article contents
Twinning and incommensurate modulation in baumoite, Ba0.5[(UO2)3O8Mo2(OH)3](H2O)~3, the first natural Ba uranyl molybdate
Published online by Cambridge University Press: 12 April 2019
Abstract
Baumoite, Ba0.5[(UO2)3O8Mo2(OH)3](H2O)~3, is a new mineral found near Radium Hill, South Australia, where it occurs in a granite matrix associated with baryte, metatorbernite, phurcalite and kaolinite. Baumoite forms thin crusts of yellow to orange–yellow tabular to prismatic crystals. The mineral is translucent with a vitreous lustre and pale yellow streak. Crystals are brittle, the fracture is uneven and show one excellent cleavage. The Mohs hardness is ~2½. The calculated density is 4.61 g/cm3. Optically, baumoite crystals are biaxial (–), with α = 1.716(4), β = 1.761(4), γ = 1.767(4) (white light); and 2Vcalc = 42.2°. Electron microprobe analyses gave the empirical formula Ba0.87Ca0.03Al0.04U2.97Mo2.02P0.03O22H11.99, based on 22 O atoms per formula unit. The eight strongest lines in the powder X-ray diffraction pattern are [dobs Å (I) (hkl)]: 9.175(39)(12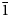 ${\bar 1}$), 7.450(100)(020), 3.554(20)(221), 3.365(31)(004, 202), 3.255(31)(123, 30
${\bar 1}$), 7.450(100)(020), 3.554(20)(221), 3.365(31)(004, 202), 3.255(31)(123, 30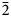 ${\bar 2}$), 3.209(28)(12
${\bar 2}$), 3.209(28)(12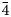 ${\bar 4}$), 3.067(33)(30
${\bar 4}$), 3.067(33)(30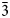 ${\bar 3}$, 222, 32
${\bar 3}$, 222, 32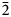 ${\bar 2}$) and 2.977(20)(142). Single-crystal X-ray studies (R1 = 5.85% for 1892 main reflections) indicate that baumoite is monoclinic, superspace group X2/m(a0g)0s with X = (0,½,0,½), with unit-cell parameters: a = 9.8337(3), b = 15.0436(5), c = 14.2055(6) Å, β = 108.978(3)°, V = 1987.25(13) Å3 and Z = 4. The crystal structure is twinned and incommensurately modulated and is based upon sheets of U6+ and Mo6+ polyhedra of unique topology. Four independent cationic sites partially occupied by Ba atoms are located between the sheets, together with H2O molecules.
${\bar 2}$) and 2.977(20)(142). Single-crystal X-ray studies (R1 = 5.85% for 1892 main reflections) indicate that baumoite is monoclinic, superspace group X2/m(a0g)0s with X = (0,½,0,½), with unit-cell parameters: a = 9.8337(3), b = 15.0436(5), c = 14.2055(6) Å, β = 108.978(3)°, V = 1987.25(13) Å3 and Z = 4. The crystal structure is twinned and incommensurately modulated and is based upon sheets of U6+ and Mo6+ polyhedra of unique topology. Four independent cationic sites partially occupied by Ba atoms are located between the sheets, together with H2O molecules.
Keywords
- Type
- Article
- Information
- Copyright
- Copyright © Mineralogical Society of Great Britain and Ireland 2019
Footnotes
Associate Editor: Ian T. Graham
References
- 3
- Cited by




