Article contents
Erssonite, CaMg7Fe3+2(OH)18(SO4)2⋅12H2O, a new hydrotalcite-supergroup mineral from Långban, Sweden
Published online by Cambridge University Press: 02 September 2021
Abstract
The new wermlandite-group mineral erssonite, ideally CaMg7Fe3+2(OH)18(SO4)2⋅12H2O (or [Mg7Fe3+2(OH)18][Ca(SO4)2]⋅12H2O), was discovered in a late-stage, low-temperature assemblage in cavities of a magnetite-bearing dolomitic rock from the Långban deposit, Värmland county, Bergslagen ore province, Sweden. The associated minerals are dolomite, calcite, members of the magnetite–magnesioferrite solid-solution series, phlogopite, chrysotile, pyroaurite and norbergite. Erssonite has a vitreous lustre and forms colourless, platy hexagonal crystals flattened on [0001], up to 0.5 mm across and up to 10 μm thick, occurring mainly as aggregates in cavities of dolomitic rock. Erssonite is malleable; separate crystals are flexible and non-elastic, with a perfect, mica-like cleavage on {0001}. The calculated density is equal to 2.02 g⋅cm–3. Raman spectroscopy shows the presence of typical bands for S–O bonds attributed to intercalated SO42– anions and structural OH– anions together with the absence of C–O bonds, attributed to carbonate anions. The chemical composition is (wt.%, electron microprobe, H2O content is calculated from structure data): MgO 28.67, CaO 2.76, Al2O3 0.23, Cr2O3 0.23, Fe2O3 16.00, SiO2 0.48, SO3 14.80, H2O 35.58, total 98.75. The empirical formula based on 38 O atoms is H41.48Ca0.52Mg7.47Fe3+2.11Al0.05Cr0.03S1.94Si0.08O38. The ideal formula is CaMg7Fe3+2(OH)18(SO4)2⋅12H2O or {Mg7Fe3+2(OH)18}{[Ca(H2O)6](SO4)2(H2O)6}. The crystal structure was determined using single-crystal X-ray diffraction data and refined to R = 0.093. Erssonite is trigonal, P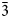 $\bar{3}$c1, with a = 9.3550(6), c = 22.5462(14) Å, V = 1708.8(2) Å3 and Z = 2. The strongest lines of the powder X-ray diffraction pattern [d, Å (I, %)(hkl)] are: 11.22 (90)(002), 5.63 (64)(004), 4.670 (100)(110, 104, 014), 2.626 (64)(032, 302), 2.435 (66)(034, 304) and 1.951 (45)(038, 308). The mineral is named in honour of the Swedish amateur mineralogist Dr. Anders Ersson (b. 1971).
$\bar{3}$c1, with a = 9.3550(6), c = 22.5462(14) Å, V = 1708.8(2) Å3 and Z = 2. The strongest lines of the powder X-ray diffraction pattern [d, Å (I, %)(hkl)] are: 11.22 (90)(002), 5.63 (64)(004), 4.670 (100)(110, 104, 014), 2.626 (64)(032, 302), 2.435 (66)(034, 304) and 1.951 (45)(038, 308). The mineral is named in honour of the Swedish amateur mineralogist Dr. Anders Ersson (b. 1971).
Keywords
- Type
- Article
- Information
- Copyright
- Copyright © The Author(s), 2021. Published by Cambridge University Press on behalf of The Mineralogical Society of Great Britain and Ireland
Footnotes
Associate Editor: Peter Leverett
References
- 7
- Cited by


