Article contents
Fluorarrojadite-(BaNa), BaNa4CaFe13Al(PO4)11(PO3OH)F2, a new member of the arrojadite group from Gemerská Poloma, Slovakia
Published online by Cambridge University Press: 28 February 2018
Abstract
The new mineral fluorarrojadite-(BaNa), ideally BaNa4CaFe13Al(PO4)11(PO3OH)F2 was found on the dump of Elisabeth adit near Gemerská Poloma, Slovakia. It occurs in hydrothermal quartz veins intersecting highly fractionated, topaz–zinnwaldite S-type leucogranite. Fluorarrojadite-(BaNa) is associated with fluorapatite, ‘fluordickinsonite-(BaNa)’, triplite, viitaniemiite and minor amounts of other minerals. It forms fine-grained irregular aggregates up to 4 cm x 2 cm, which consist of individual anhedral grains up to 0.01 mm in size. It has a yellowish-brown to greenish-yellow colour, very pale yellow streak and a vitreous to greasy lustre. Mohs hardness is ~4½ to 5. The fracture is irregular and the tenacity is brittle. The measured density is 3.61(2) g cm–3 and calculated density is 3.650 g cm–3. Fluorarrojadite-(BaNa) is biaxial (+) and nonpleochroic. The calculated refractive index based on empirical formula is 1.674. The empirical formula (based on 47 O and 3 (OH + F) apfu) is A1(Ba0.65K0.35)Σ1.00 A2Na0.35 B1(Na0.54Fe0.46)Σ1.00 B2Na0.54Ca(Ca0.74Sr0.20Pb0.02Ba0.04)Σ1.00Na2 Na3Na0.46 M(Fe7.16Mn5.17Li0.37Mg0.12Sc0.08Zn0.06Ga0.02Ti0.02)Σ13.00 Al1.02P11O44PO3.46(OH)0.54 W(F1.54OH0.46). Fluorarrojadite-(BaNa) is monoclinic, space group Cc, a = 16.563(1) Å, b = 10.0476(6) Å, c = 24.669(1) Å, β = 105.452(4)°, V = 3957.5(4) Å3 and Z = 4. The seven strongest reflections in the powder X-ray diffraction pattern are [dobs in Å, (I), hkl]: 3.412, (21), 116; 3.224, (37), 206; 3.040, (100), 42 $\bar 4$; 2.8499, (22), 33
$\bar 4$; 2.8499, (22), 33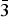 $\bar 3$; 2.7135, (56), 226; 2.5563, (33), 028 and 424; 2.5117, (23), 040. The new mineral is named according to the nomenclature scheme of arrojadite-group minerals, approved by the IMA CNMNC. In fluorarrojadite-(BaNa), Fe2+ is a dominant cation at the M site (so the root-name is arrojadite) and two suffixes are added to the root-name according to the dominant cation of the dominant valence state at the A1 (Ba2+) and B1 sites (Na+). A prefix fluor is added to the root-name as F– is dominant over (OH)– at the W site.
$\bar 3$; 2.7135, (56), 226; 2.5563, (33), 028 and 424; 2.5117, (23), 040. The new mineral is named according to the nomenclature scheme of arrojadite-group minerals, approved by the IMA CNMNC. In fluorarrojadite-(BaNa), Fe2+ is a dominant cation at the M site (so the root-name is arrojadite) and two suffixes are added to the root-name according to the dominant cation of the dominant valence state at the A1 (Ba2+) and B1 sites (Na+). A prefix fluor is added to the root-name as F– is dominant over (OH)– at the W site.
Keywords
- Type
- Article
- Information
- Copyright
- Copyright © Mineralogical Society of Great Britain and Ireland 2018
Footnotes
Associate Editor: G. Diego Gatta
References
- 7
- Cited by


