Article contents
Evaluation of the Plasticity of Novel Regulatory Cells—Telocytes—in the Gonad of the Male Chinese Soft-Shelled Turtle (Pelodiscus sinensis) Associated with Seasonal Reproductive Activity
Published online by Cambridge University Press: 07 October 2022
Abstract
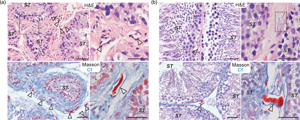
Telocyte (TC)—a new type of interstitial cell with long telopodes, can form cellular junctions with various tissues or cells to participate in the regulation of multitudes of physiological activities and diseases. This study aimed to characterize the morphology, molecular features, and potential functions of hormone regulation in Chinese soft-shelled turtle (Pelodiscus sinensis) testis TCs at different reproductive stages by histological evaluation, immunohistochemistry (IHC), immunofluorescence (IF), and transmission electron microscopy. During hibernation, TCs were widely distributed in the interstitial tissue. In contrast, during reproductive activity, TCs were noted to be in close proximity with peritubular myoid cells surrounding the seminiferous tubule. Moreover, formed cell–cell junctions were observed between TCs and PTMs. The results of IHC and IF showed that the immunophenotype of testicular TCs in hibernating Chinese soft-shelled turtles is CD34+Vimentin−, while the reproductive telopodes (Tps) show low expression of vimentin. The androgen receptor is expressed in Tps of TCs of testis during hibernation. Our results showed also that TCs in seasonal breeding animals regulate the activity of neighboring cells by releasing extracellular microvesicles (EXMVs), thus influencing the activity of spermatogenesis and steroidogenesis. Consideration of our novel and interesting results indicate that the whole area warrants further research.
- Type
- Biological Applications
- Information
- Copyright
- Copyright © The Author(s), 2022. Published by Cambridge University Press on behalf of the Microscopy Society of America
Footnotes
These authors contributed equally.
References
- 2
- Cited by





