Article contents
Comparative Cellular Localization of Sugar Residues in Bull (Bos taurus) and Donkey (Equus asinus) Testes Using Lectin Histochemistry
Published online by Cambridge University Press: 12 October 2021
Abstract
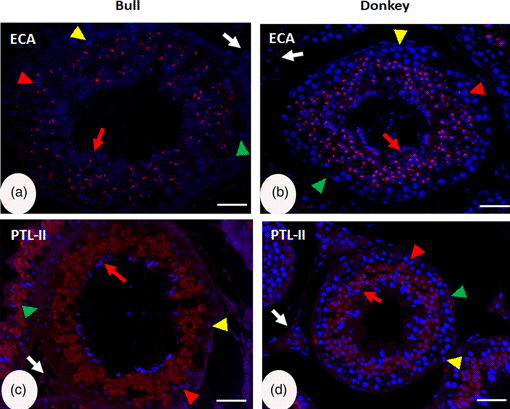
Lectins are glycoproteins of a non-immune origin often used as histochemical reagents to study the distribution of glycoconjugates in different types of tissues. In this study, we performed a comparative cellular localization of sugar residues in bull and donkey testes using immunofluorescent lectin histochemistry. We inspected the cellular localization of the glycoconjugates within the testes using 11 biotin-labeled lectins (LCA, ConA, PNA, WGA, DBA, SBA, ECA, BPL, PTL-II, UEA-1, and PHA-E4) classified under six groups. Although the basic testicular structure in both species was similar, the cellular components showed different lectin localization patterns. The statistical analysis revealed no significant association between the intensity of labeling and different variables, including group and type of lectin and type of cell examined, at p < 0.05. However, a stronger response tended to occur in the donkey than in the bull testes (odds ratio: 1.3). These findings may be associated with the different cellular compositions of the glycoproteins and modification changes during spermatogenesis. Moreover, glycoconjugate profiling through lectin histochemistry can characterize some cell-type selective markers that will be helpful in studying bull and donkey spermatogenesis.
- Type
- Biological Applications
- Information
- Copyright
- Copyright © The Author(s), 2021. Published by Cambridge University Press on behalf of the Microscopy Society of America
References
- 2
- Cited by





