INTRODUCTION
Chlorurus sordidus is a marine species that sometimes enters brackish water and lives in association with coral reefs (Riede, Reference Riede2004) at depths from the surface down to 50 m (Fischer et al., Reference Fischer, Sousa, Silva, de Freitas, Poutiers, Schneider, Borges, Feral and Massinga1990). It reaches maturity at total length 150 mm (Randall et al., Reference Randall, Allen and Steene1990). Similarly, Hipposcarus harid has the same mode of life, but lives at a depth range of 1–25 m (Lieske & Myers, Reference Lieske and Myers1994). It reaches maturity at 750 mm total length (Randall, Reference Randall, Smith and Heemstra1986).
Otolith morphology has proven useful in phylogenetics and palaeoecology, yet such studies for the family Scaridae are scarce. Prominent studies have been conducted in the Indo-Pacific region, North America, China and the Red Sea. Nolf (Reference Nolf1985) provided a diagram of the sagittal otolith of Scarus croicensis (= S. iseri) (Bloch, 1789). Weisler (Reference Weisler1993) commented on the importance of the otoliths of Scarus perspicillatus (Steindachner, 1879) for archaeological studies, indicating their significance in determining their communities during prehistoric periods. Smale et al. (Reference Smale, Watson and Hecht1995) described six species of the genus Scarus and of the species Calostomus spinidens, Leptoscarus vaigiensis and Hipposcarus harid, demonstrating the use of otoliths in phylogeny. Rivaton & Bourret (Reference Rivaton and Bourret1999) published images of otoliths of nine species of the genus Scarus and two species of the genus Cetoscarus from the Indo-Pacific region, showing an innovative technique for otolith morphological studies. Tuset et al. (Reference Tuset, Lombarte and Assis2008) briefly described the otolith of Saprisoma cretense (Linnaeus, 1758) and S. rubripinne (Valenciennes, 1840), showing their superiority in phylogenetic studies compared with genetic studies. Baremore & Bethea (Reference Baremore and Bethea2010) used otoliths of Nicholsina usta (Valenciennes, 1840) from the Gulf of Mexico. Lin & Chang (Reference Lin and Chang2012) described the otoliths of seven species of the genus Scarus and species of the genera Calotomus, Chlorurus, Hipposcarus and Leptoscarus from Taiwan, confirming their phylogenetic relations, and Sadighzadeh et al. (Reference Sadighzadeh, Tuset and Valinassab2012) gave short descriptions of two Scarus species using otoliths morphology. Nolf (Reference Nolf2013) produced otolith diagrams of Nicholsina usta (Valenciennes, 1840) and Scarus croicensis Bloch, 1790, showing their phylogenetic relationships. Except for the description of the otolith of H. harid from the Red Sea by Smale et al. (Reference Smale, Watson and Hecht1995), there is a gap in information on the morphology of scarid otoliths from the Red Sea.
Distinguishing between C. sordidus and H. harid using external morphological traits is a challenge. Using otolith morphology, however, may prove reliable in making such a distinction. Additionally, otolith morphology may prove a useful supporting tool for archaeology, and palaeoichthyology (Nolf, Reference Nolf1985). The objective of this study is to describe otoliths of juveniles and adults of fish of the family Scaridae. Collections were from the Red Sea, due to the high importance of this species in the region. This work will help resolve the taxonomy among the members of the family Scaridae.
MATERIALS AND METHODS
The vicinity of Hurghada was chosen as it represents one of the main fishing grounds for the two study species. Understanding the asymmetry of the two species is important to show the effect of this phenomenon on the settlement of their larvae in this important fishing ground, as has been shown for other fish species (Battaglia et al., Reference Battaglia, Malara, Romeo and Andaloro2010). Specimens of parrotfish were obtained during the fishing season 2012–2013 through monthly sampling from the commercial landings. The fish were caught using gill nets of 60–100 m long with mesh size of 2 (0.25 cm). Standard length (TL) was measured to the nearest 0.1 cm using a digital caliper. The specimens of C. sordidus range from 161 to 250 mm in total length (N = 123). They were placed in seven groups according to their total length: Group I, 161–170 mm, N = 10; Group II, 171–180 mm, N = 15; Group III, 181–190 mm, N = 20; Group IV, 201– 210 mm, N = 15; Group V, 211– 220 mm, N = 25; Group VI, 231–240 mm, N = 15; and VII, 241–250 mm, N = 15. The specimens of H. harid, which range from 140 to 310 mm in total length (N = 115). Otoliths with obvious evidence of calcite crystallization or other abnormal formations were not considered in this study.
Fish otoliths are located on the two sides of the basioccipital bone and separated by a thin septum arising from the midventral ridge of the occipital (Ruck, Reference Ruck1976; Jawad et al., Reference Jawad, Al-Jufaili and Al-Shuhaily2007; Jawad, Reference Jawad2008). Otoliths were removed by slicing open the top of the cranium and exposing the brain. After removal of the brain with a sharp scalpel, the otic capsules were separated and the otoliths gently removed with a pair of fine tweezers. Later, the otoliths were cleaned with 70% ethanol and stored dry in a small glass tube. Scanning electron microscopy was used to record the morphological characteristics on the inner face of the saccular otolith (sagitta). The terminology of otolith morphology follows Smale et al. (Reference Smale, Watson and Hecht1995) (Figure 1). The otoliths to be used for SEM were air dried and mounted on an aluminium stub using double-sided carbon tape. Otolith left sagitta were viewed in an ESEM Fei Quanta 200 at 10.0–20.0 KV.
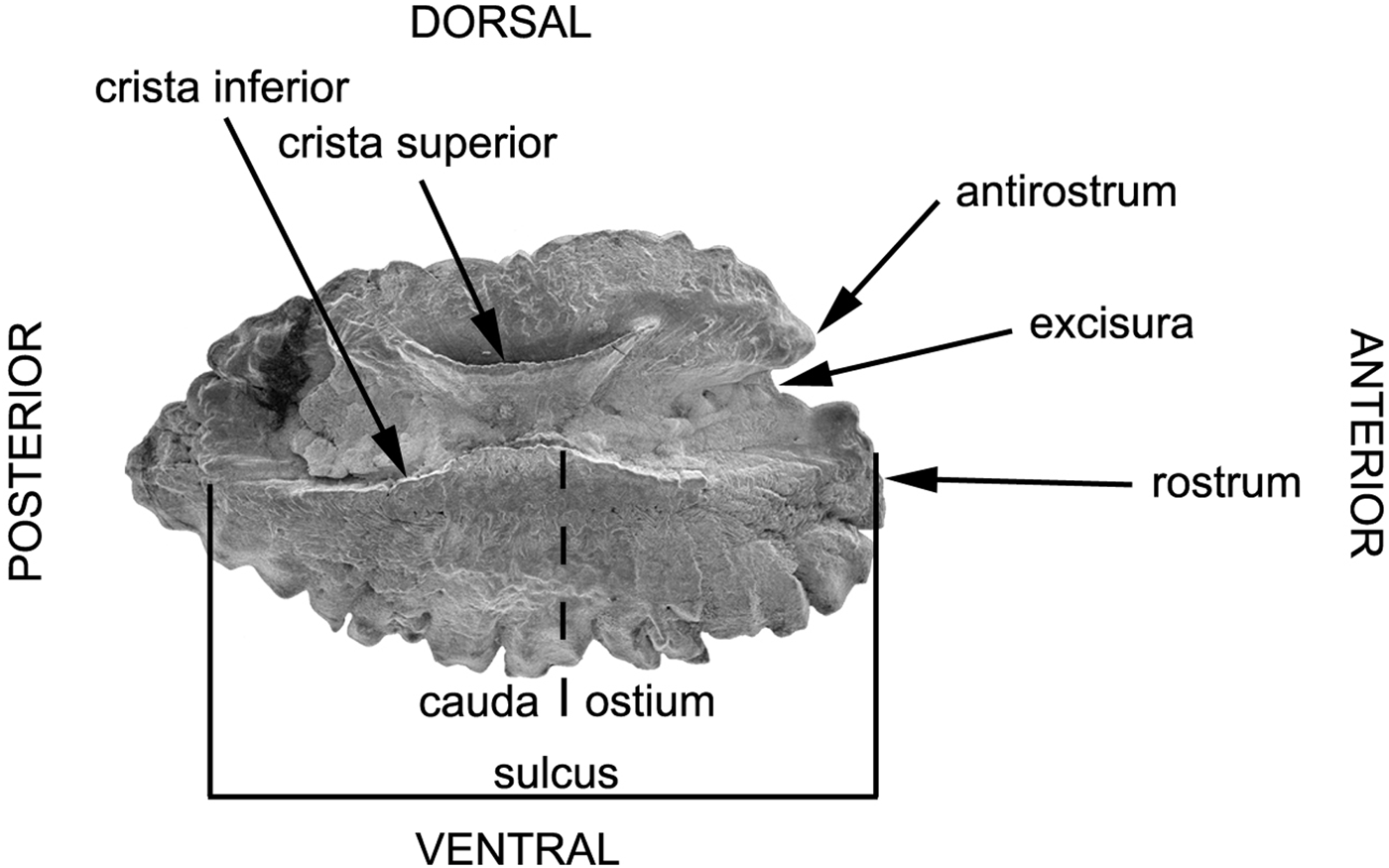
Fig. 1. Diagram of the mesial surface of the left otolith of Hipposcarus harid illustrating various features of the otolith, which are described in the text.
Otolith morphometry
According to the terminology of Avigliano et al. (Reference Avigliano, Martinez and Volpedo2014, Reference Avigliano, Jawad and Volpedo2015), a number of otolith measurements were recorded. These were otolith length (OL, mm), otolith width (OW, mm), otolith perimeter (OP, mm), otolith surface (OS, mm2), sulcus perimeter (SP, mm), sulcus surface (SS, mm2), sulcus length (SL, mm), cauda length (CL, mm), ostium length (OSL, mm), rostrum width (RW, mm) and rostrum length (RL, mm) (Figure 2). These measurements were taken for all the otoliths using image-processing systems (image analysis software TpsDig2; Rohlf, Reference Rohlf2006). Subsequently, the following otolith shape indices were calculated: aspect ratio (OW/OL, %), percentage of the otolith surface occupied by the sulcus (SS/OS, %), percentage of the sulcus length occupied by the cauda length (CL/SL, %), percentage of the sulcus length occupied by the ostium length (OSL/SL, %), rostrum aspect ratio (RW/RL, %), and percentage of the rostrum length occupied by the otolith length (RL/OL, %). CL/SL and OSL/SL indices were used for the first time in this work.
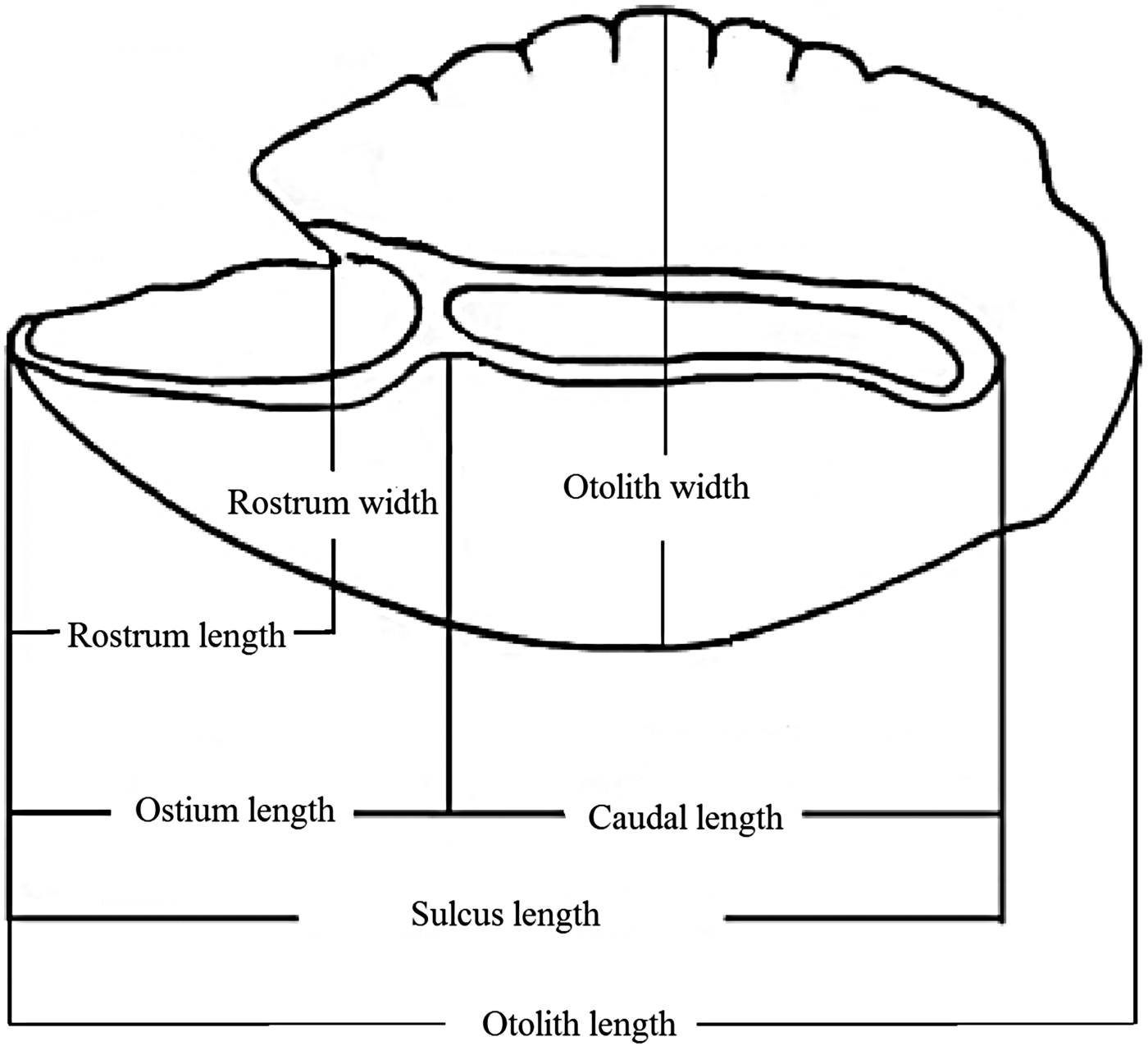
Fig. 2. Generalized scheme of the inner surface of saccular otoliths of a parrotfish illustrating otolith measurements, described in the text.
RESULTS
There were ontogenetic changes in the otoliths of the two scarid fish species. In the otoliths of C. sordidus (Figure 3), otolith width, otolith depth, mesial surface shape, lateral surface shape, shape of sulcus acusticus, column, rostrum, and size of rostrum were similar. The otoliths of the small adults (GI) C. sordidus differed from the large adult ones in 14 out of the 22 characteristics studied. Detailed descriptions of the otoliths in seven size classes of fish are shown in Table 1.

Fig. 3. (A–J) Otoliths of Chlorurus sordidus. (A) 161 mm TL; (B) 175 mm TL; (C) 183 mm TL; (D) 184 mm TL; (E) 204 mm TL; (F) 210 mm TL; (G) 215 mm TL; (H) 220 mm TL; (I) 240 mm TL; (J) 248 mm TL.
Table 1. Otolith characteristics of seven size classes of Chlorurus sordidus (Figure 3).
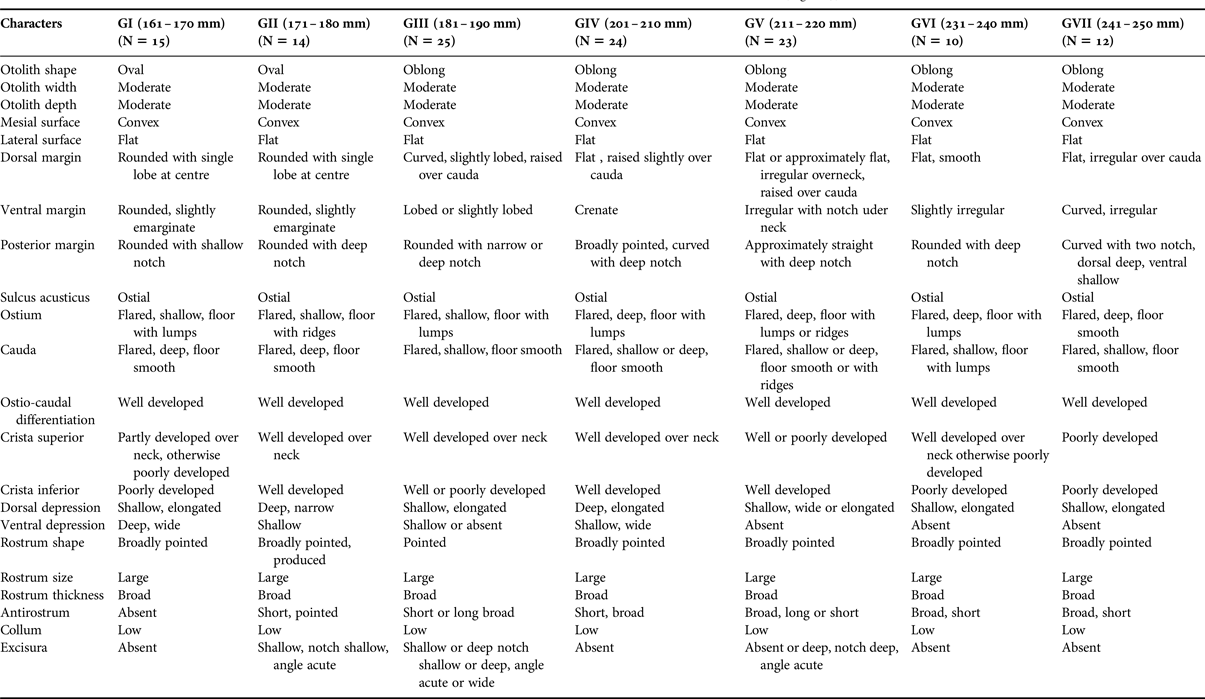
Number of otoliths (N) examined in parantheses.
In the otoliths of H. harid, otolith width, otolith depth, mesial (inner) and lateral surface shapes, shape of sulcus acusticus, rostrum and size of rostrum were similar. The otoliths of the very small (GI) H. harid differ from the large-sized ones in 13 out of the 22 characteristics studied. In the otoliths of H. harid, the following characters were shown to be not different among young individuals studied: otolith shape, width and depth, mesial surface shape, lateral surface shape, shape of sulcus acusticus, rostrum, ventral depression and size of rostrum. Detailed descriptions of the otoliths in the seven size classes of fish are shown in Figure 4 and Table 2.

Fig. 4. (A–H) Otoliths of Hipposcarus harid. (A) 140 mm TL; (B) 160 mm TL; (C) 222 mm TL; (D) 245 mm TL; (E) 263 mm TL; (F) 270 mm TL; (G) 288 mm TL; (H) 306 mm TL.
Table 2. Otolith characteristics of seven size classes of Hiposarcus harid (Figure 4).
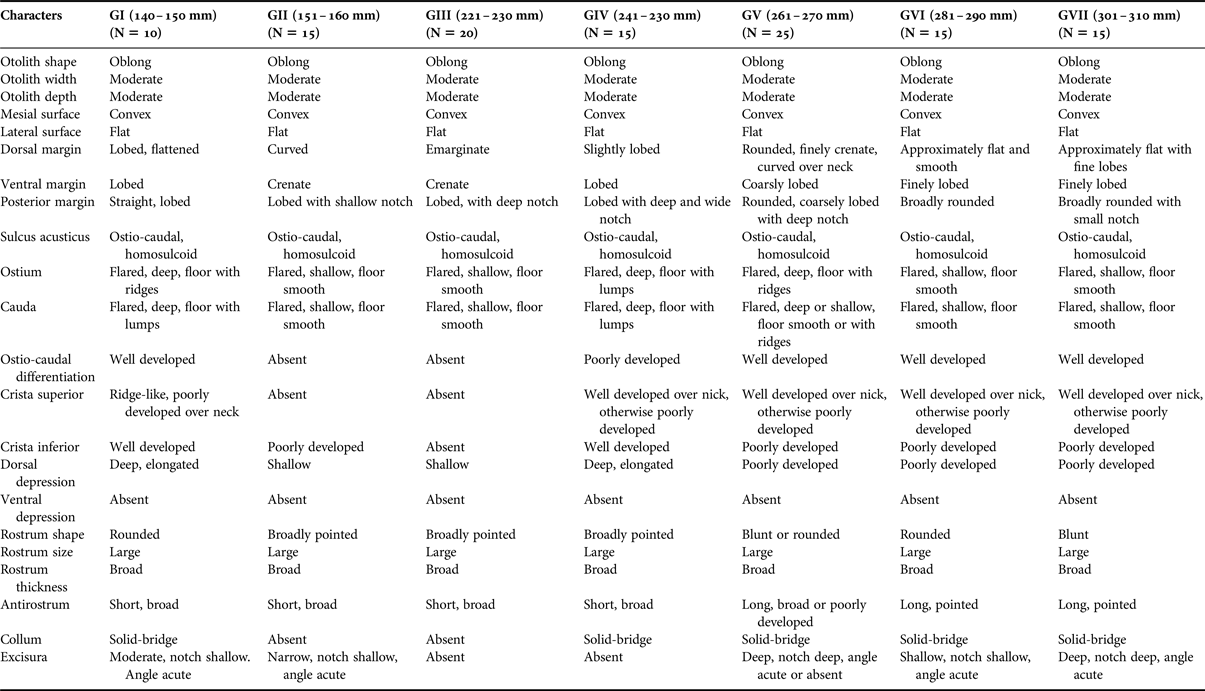
Number of otolith (n) examined in parantheses.
The shape of the otoliths of C. sordidus can be either oval or oblong, while they are oblong in H. harid (Tables 1 & 2, Figures 3 & 4).
The mesial surface, however, provided a wide range of ornamental sculptures that in most cases helped to differentiate taxa.
The shape of the dorsal margin of the otolith of C. sordidus is mainly rounded with lobes in GI to GIII and becomes flat in GIV to GVII. In the otoliths of H. harid, it varies between lobed, curved and emarginate in GI to GV and becomes approximately flat in GVI and GVII. Lobes and irregularities are present on the dorsal margin in fish groups I to V, which becomes smooth or nearly smooth in GVI and GVII.
The ventral margin in the otoliths of C. sordidus was generally rounded in GII and GI and became lobed or irregular in adult fish groups. On the other hand, in H. harid, it was generally lobed in otoliths of young and adult with slight variations in the size of the lobes in the different length groups.
The posterior margin in otoliths of C. sordidus were usually rounded in groups of smaller length and always rounded in the groups of larger length. The presence of a notch was a persistent feature of this margin. It was shallow in the otoliths of small adult fish and deep in large adult fish. Two notches were observed in otoliths of GVII. Presence of lobes and a deep notch were two features of the otoliths of GI–GIV of H. harid. This margin became rounded and broadly rounded with a shallow notch in otoliths of large-sized young fish (GV to GVII).
The ostium in otoliths of C. sordidus of all length groups studied was characterized to be flared and its floor covered with lumps. The depth of the ostium changed from shallow in GI and GIII to deep in the otoliths of the large-sized young fish (GIV–GVII). In otoliths of H. harid, the ostium was also flared in all length groups studied. Features of otoliths of GI–GV were the deep sulcus, with its lower side covered by lumps and sometimes with ridges. The sulcus became shallow and its floor smooth in large-sized young fish groups (GVI–GVII).
The cauda in otoliths of all length groups of both C. sordidus and H. harid was flared and deep, its lower side was mainly smooth and sometimes covered by lumps or ridges. The column was often well developed in otoliths of the large-sized fish groups in both species.
The crista superior of the ostium and the cauda was generally poorly developed, but usually well developed over the neck in otoliths of C. sordidus, where it became a high ridge, bordering a dorsal depression. The same applied to the crista superior in otoliths of H. harid (GIV–GVII), where it was generally less developed in otoliths of small-sized adult fishes (GI and GIII).
The crista inferior was less defined in the otoliths of small-sized adult fishes of C. sordidus (GI); it became well developed in GII–GVII. On the contrary, the crista inferior in H. harid was better developed in otolith of small-sized young fishes (GI) and showed some variation in groups II–VII.
The dorsal depression in both species varied between shallow or deep and elongated or wide in otoliths of young fish (GI–GIV). It was very pronounced in otoliths of large-sized young fish (GV–GVII), accentuated by the high crista superior on its ventral side. There was no ventral depression in either scarid species.
The anterior rim was rounded or straight, with a distinct rostrum and excisura in most specimens of both species, although sometimes less distinct in C. sordidus. The shape of the rostrum (broadly pointed, blunt, rounded) was characteristic in otoliths of the small-sized adult fishes of both C. sordidus and large-sized young fishes of H. harid, but it became broadly pointed and rounded in larger fish sizes of C. sordidus and H. harid respectively. The antirostrum varied in shape between complete absence or short or broad in otoliths of small-sized adult fishes of C. sordidus (GI–GV), but remained broad and short in large-sized fish groups (GVI and GVII). However, it was short and broad in otolith of the small-sized young fishes of H. harid (GI–GV), but became long and pointed in the two length groups of larger young fishes (GVI and GVII). The antirostrum was, however, always shorter than the rostrum. Slight variations (absence, shallow, deep, notch deep or shallow, angle acute) were noticed in the excisura of the otoliths of small adult fishes of C. sordidus (GI–GIV), it was sometimes absent in the two large adult fish groups (GVI and GVII). Variations in the shape (absence, shallow, deep, notch wide, shallow, angle acute) of the excisura of the otoliths of H. harid remained consistent throughout the different fish length groups studied.
Lower proportions were found in CL and RL for C. sordidus and H. harid respectively, while higher proportions were observed in OL and OP for C. sordidus and H. harid respectively. As for the percentage indices, the lowest values were shown in CL/SL and RW/RL and the highest were for CL/SL in both species studied (Table 3).
Table 3. Mean and standard deviation and range (minimum–maximum) of the morphological indices of Chlorurus sordidus and Hipposcarus harid.
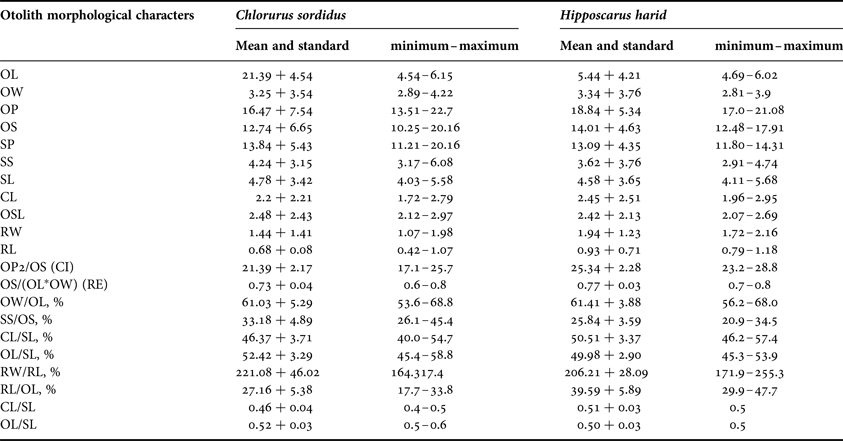
OL, otolith length; OW, otolith width; OP, otolith perimeter; OS, otolith surface; SP, sulcus perimeter; SS, sulcus surface, SL, sulcus length; CL, cauda length; OSL, ostium length; RW, rostrum width; RL, rostrum length; CI, circularity; RE, rectangularity.
For C. sordidus, the non-linear power function coefficient ‘b’ of otolith length (Y) on different otolith variables (X) was highest for OL/OP, where the values were 4.370. For H. harid, the value of the coefficient ‘b’ was 6.698 for OL/SP. The lowest values for both species were 0.034 and 0.426 for OL/RL and OL/OSL for C. sordidus and H. harid respectively.
The highest correlations found of the otolith length with the different otolith indices is r = 0.87 and r = 0.86 for OL/SS and OL/OP for C. sordidus and H. harid respectively.
The growth of the different otolith morphometric characters relative to the otolith length of C. sordidus were shown to be negative for OL/OW and OL/OP. The other relationships were shown to have a positive allometric growth. For H. harid, the relationships OL/OW, OL/OP/OL/SP, and OL/RW had negative allometric growth and the remaining six relationships had positive allometric growth (Table 4).
Table 4. Statistical relationship of morphometric traits of Chlorurus sordidus and Hipposcarus harid.
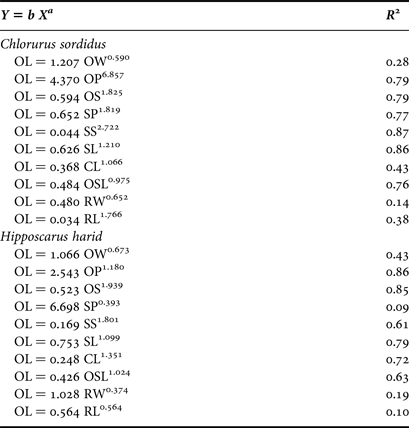
OL, otolith length; OW, otolith width; OP, otolith perimeter; OS, otolith surface; SP, sulcus perimeter; SS, sulcus surface, SL, sulcus length; CL, cauda length; OSL, ostium length; RW, rostrum width; RL, rostrum length; R 2, correlation coefficient.
DISCUSSION
Since the early 20th century, certain characters of the saccular otolith have been used to support taxonomic studies (e.g. Chaine & Duvergier, Reference Chaine and Duvergier1934) despite the degree of inter-specific variation (Jawad et al., Reference Jawad, Al-Jufaili and Al-Shuhaily2007). The present study of otolith morphology in two scarid species considered a wide range of otolith characteristics. However, only a few characteristics were taxonomically important for these species, and useful in systematics.
The results of the present study showed two groups of characteristics: (1) characteristics that are consistent in the otoliths of fish from different length groups, which are thus valuable to identify the species and (2) characteristics that vary due to ontogenetic changes, but that may be useful to define developmental stages.
Morphology and external colouration are used to recognize several species of parrotfish. Such groupings are consistent with the phylogenetic evolution in their family (Bellwood, Reference Bellwood1994). Larger eyes and bright colours are characteristics of species living in shallow waters. Such criteria assist the fish in visual communication. The otoliths of these species are usually small so they can avoid background noise produced by rough seas (Lombarte et al., Reference Lombarte, Palmer and Matallanas2010). On the contrary, a darker colouration is characteristic of species inhabiting deeper or dimly illuminated waters in which they possess larger otoliths (Lombarte et al., Reference Lombarte, Palmer and Matallanas2010). This pattern was clearly noted in the two scarid species studied, illustrating the relationship of morphology, external colouration and otolith size. The two species studied are pelagic, live in water depths ranging from the surface down to 50 m (Froese & Pauly, Reference Froese and Pauly2016) and have small otoliths. These observations are in agreement with those of snappers from the Persian Gulf (Sadighzadeh et al., Reference Sadighzadeh, Otero-Ferrer, Lombarte, Fatemi and Tuset2014). Differences in colour are also important in sympatric, brightly coloured species as it is an essential component of sexual selection. Notothenoid species have similar relationships between otolith size, habitat and behaviour (Klingenberg & Ekau, Reference Klingenberg and Ekau1996; Lombarte et al., Reference Lombarte, Torres and Morales-Nin2003). The development of the antero-dorsal area of the sagittal otolith can be related to the behaviour of the fish.
Sadighzadeh et al. (Reference Sadighzadeh, Otero-Ferrer, Lombarte, Fatemi and Tuset2014) found such a relationship in the variations of snapper otolith.
The results of the present study show that there are variations in the overall morphology of the otoliths from small-sized adult individuals of C. sordidus and small-sized young fishes of H. harid. Out of 22 otolith characters examined, C. sordidus and H. harid have 13 and 14, respectively, which are similar between young and adult. Such variation may be related to the organic matrix and the way in which CaCO3 is deposited during sagittal development (Volpedo & Echevarria, Reference Volpedo and Echevarria2003). In the species studied here, calcium is not uniformly deposited as the otolith grows, judging from the inconsistency of shape throughout the ontogenetic development of the two species. This result agrees with the work of Smale et al. (Reference Smale, Watson and Hecht1995) on H. harid of 325 and 424 mm, and with the descriptions of other scarid species (Rivaton & Bourret, Reference Rivaton and Bourret1999, on S. rivulatus of 291 mm; Lin & Chang, Reference Lin and Chang2012, on H. longiceps of 203 mm; Sadighzadeh et al., Reference Sadighzadeh, Tuset and Valinassab2012, on S. ghobban of 191 mm).
The two scarid species studied shared similar patterns in otolith morphology from different fish length groups. These characters cannot be used as a tool to separate these species from other members of the family Scaridae as members of other scarid genera also share them.
The shape of the otolith varies between oval and oblong in C. sordidus, but is oblong in the otoliths of both small- and large-sized fishes of H. harid. In the case of C. sordidus, there are only two shapes and with such variation it is possible to separate the otoliths of the small-sized adult (GI and GII) from the large-sized adult. For H. harid, it is possible to determine a predictable shape for its otolith. Such consistency can be used as a trait for identifying this species. Such a result does not agree with that of Smale et al. (Reference Smale, Watson and Hecht1995) on the same species.
Three margins of the otolith, dorsal, ventral and posterior, show a wide range of shape variation in the two scarid species studied. There are grades of lobation and irregularities in their shapes throughout the different length groups. This result is in agreement with those obtained for triplefin (Enneapterygius spp.) by Jawad (Reference Jawad2008) and Jawad et al. (Reference Jawad, Al-Jufaili and Al-Shuhaily2007) on Saurida tumbil.
The shape of both cristae superior and inferior of the otoliths of the two scarid species cannot be considered as a criterion to separate the otoliths of fishes from different length groups. The shape and nucleus location result from the release of soluble Ca2+ on the proximal side (Ibsch et al., Reference Ibsch, Anken and Rahmann2004), which in turn precipitates as CaCO3 crystals due to an increasing alkaline gradient, from the sulcal area towards the otolith edge (Gauldie & Nelson, Reference Gauldie and Nelson1990). As a result, the growth of the crista superior and crista inferior is privileged and there is a more important development of the sulcal side. The macula is elongated and narrow in teleosts, and the cristae superior and inferior are proportionally more important than the colliculum (Ladich & Popper, Reference Ladich and Popper2001). The macula faces the column, and prevents otolith growth at this level (Pannella, Reference Pannella, Rhoad and Lutz1980; Popper & Hoxter, Reference Popper and Hoxter1981). This is clear in some species where the column is either poorly developed or absent. Lombarte et al. (Reference Lombarte, Torres and Morales-Nin2003) showed the variability in the shape of the sagitta in Merluccius as a relation to genetic, ontogenetic and environmental factors. Many previous studies on otoliths from fossil and extant fish have demonstrated that the sulcus morphology usually is consistent among the species of a single genus (Nolf, Reference Nolf1985), and thus this feature is probably controlled genetically (Gauldie, Reference Gauldie1988).
However, sulcus variation has been shown to be related with specialization in hearing abilities, and thus interspecific sulcus variation may result from ecomorphological adaptations (Ramcharitar et al., Reference Ramcharitar, Deng, Ketten and Popper2004; Popper et al., Reference Popper, Ramcharitar and Campana2005).
Apart from sulcus morphology, a correlation between particular otolith features (e.g. rostrum, antirostrum proportions) and biological functions such as swimming ability, feeding or other activities has not yet been established (Popper et al., Reference Popper, Ramcharitar and Campana2005). Considering the great diversity of teleost fishes there might be some correlation between the otolith rostrum length and swimming ability (Volpedo & Echevarria, Reference Volpedo and Echevarria2003), but this has not been shown to be significant in the discrimination of closely related species (Reichenbacher et al., Reference Reichenbacher, Sienknecht, Küchenhoff and Fenske2007). In the present study, the general morphology of the rostrum is broad, long and pointed in C. sordidus, and large, broad, rounded or blunt in H. harid. Such consistency in these features agrees with the finding of Reichenbacher et al. (Reference Reichenbacher, Sienknecht, Küchenhoff and Fenske2007).
The shape of the excisura in the otoliths of fish of different length groups of both C. sordidus and H. harid is not considered to be a good taxonomic character to separate the two species from their congeners.
Significant variations in the morphology of the otoliths of C. sordidus and H. harid were observed. These variations exist in specimens taken from close geographic regions. Smale et al. (Reference Smale, Watson and Hecht1995) described the morphology of C. sordidus from Dahab, Sinai, Red Sea, and H. harid from the Red Sea. Their descriptions cover different aspects of the otolith morphology and are not restricted to certain criteria. Changes in physical and chemical parameters may be responsible for differences in calcareous concrescence formation in fishes (Kumar et al., Reference Kumar, Chakraborty and Jaiswar2012). The presence of a cauda and an ostium with different textures, where the cauda curves to the ventral margin and is much deeper than the ostium have also been reported (Correa & Vianna, Reference Correa and Vianna1993). Baldas et al. (Reference Baldas, Perez Macri, Volpedo and Echeverria1997) have reported a similar calcareous concrescence and a dorsal depression in the inner face. The selective pressures act on sagittae so that their morphology meets specific auditory needs (Platt & Popper, Reference Platt, Popper, Tavolga, Popper and Fay1981; Popper & Coombs, Reference Popper and Coombs1982; Gauldie, Reference Gauldie1988). Some otolith size differences are related to fish growth, but otoliths in very large species can be much smaller than those of small species and vice versa (Campana, Reference Campana2004). A limited comparison made by Friedland & Reddin (Reference Friedland and Reddin1994) suggested greater genetic influences on otolith shape.
The geographic variations observed in the morphology of the otoliths of C. sordidus are in agreement with the findings herein. Rivaton & Bourret (Reference Rivaton and Bourret1999) showed an image of an otolith of a specimen of C. sordidus (235 mm) with the following set of characters. These characters were compared with those of the otoliths obtained from fish of the same length group in our sample. The characters were: the dorsal margin is lobed vs flat and smooth, ventral margin lobed vs slightly irregular, posterior margin straight with notch vs rounded, cauda deep vs shallow and excisura shallow, notch wide and angle acute vs absent. The descriptions of the otolith in Rivaton & Bourret (Reference Rivaton and Bourret1999) and of those in the present paper provide an integrated description of the otolith of this species.
The results from our analysis also demonstrate intraspecific variations in otolith shape for each species. This suggests that a genetic flow within populations of both species. The overall data collected in this study indicate that variability in otolith shape can be added to the traits describing each species. Bani et al. (Reference Bani, Poursaeid and Tuset2013) reached a similar conclusion in their study on gobiids from the Caspian Sea. Further investigation is required, including a comparative study of the shape and geometry of the sagittal otolith, to add further taxonomic characters for the identification of these species.
ACKNOWLEDGEMENTS
Our sincere thanks are due to Ralf Riedel, Gulf Coast Research Laboratory, University of Southern Mississippi, USA, for reading the manuscript and for his valuable advice and suggestions, and to Julien CILLIS, Royal Belgian Institute of Natural Sciences, for the production of the EM images.










