Introduction
Early cognitive development underlies individual differences in children's behavior and academic performance (Cooper & Farran, Reference Cooper and Farran1988; Rohde & Thompson, Reference Rohde and Thompson2007; see Tramontana, Hooper, & Selzer, Reference Tramontana, Hooper and Selzer1988, for a review). In the developing world, poverty, disease and malnutrition inhibit children from attaining their developmental potential. Several studies suggest that worms, affecting approximately 90 million school-age children (Brooker, Clements, & Bundy, Reference Brooker, Clements and Bundy2006), impair cognitive functions (e.g., Boivin et al., Reference Boivin, Giordan, Ndanga, Makakala, Manzeki, Ngunu and Kibungu1993; Ezeamama et al., Reference Ezeamama, Friedman, Acosta, Bellinger, Langdon, Manalo and McGavey2005; Jukes et al., Reference Jukes, Nokes, Alcock, Lambo, Kihamia, Ngorosho and Bundy2002; Nokes et al.,Reference Nokes, Grantham-McGregor, Sawyer, Cooper, Robinson and Bundy1992, Reference Nokes, McGarvey, Shiue, Guanling, Hawai, Bundy and Olds1999; Sakti et al., 1999).
Worms also infect approximately a third of pregnant women in Sub-Saharan Africa (Bundy, Chan, & Savioli, Reference Bundy, Chan and Savioli1987). We hypothesized that maternal worm infections might influence the development of cognitive function in infants. Animal studies suggest that by depleting nutrients available to the fetus, maternal worm infections may interfere with processes such as myelination and the development of neurotransmitter systems vital for neurological and cognitive functioning (Beard et al., Reference Beard, Unger, Bianco, Paul, Rundle and Jones2007). Beard et al. (Reference Beard, Hendricks, Perez, Murray-Kolb, Berg, Vernon-Feagans and Tomlinson2005) found that offspring of iron-deficient rats had lower iron content, lowered activity in the dopamine transporter system in the caudate nucleus and substantia nigra, reduced motor activity, and higher anxiety levels than controls. In humans, dopamine influences the limbic system and frontal cortex: disruptions in the dopamine system interfere with psychomotor and executive function (Goto & Grace, Reference Goto and Grace2005; Smith & Kieval, Reference Smith and Kieval2000). Hence, we postulated that early human executive function might be particularly vulnerable to effects of maternal worm infections. Executive function comprises processes involved in goal-directed behavior or planning and consists of complementary sub-skills including working memory, inhibitory control and attentional flexibility (Hughes, Reference Hughes1998; Miyake et al., Reference Miyake, Friedman, Emerson, Witzki, Howerter and Wager2000).
Maternal worm infections co-exist with several important potential confounders, such as maternal under-nutrition (Goldenberg, Hoffman, & Cliver, Reference Goldenberg, Hoffman and Cliver1998; Hack, Reference Hack1998), maternal infections, including HIV and malaria (Gay et al., Reference Gay, Armstrong, Cohen, Lai, Hardy, Swales and Scott1995; Gentile, Boll, Stagno, & Pass, Reference Gentile, Boll, Stagno and Pass1989) and exposures in infancy including malaria (Dugbartey & Spellacy, Reference Dugbartey and Spellacy1997), recurrent diarrhea (Berkman, Lescano, Gilman, Lopez, & Black, Reference Berkman, Lescano, Gilman, Lopez and Black2002), anemia (Clarke, Grantham-McGregor, & Powell, Reference Clarke, Grantham-McGregor and Powell1991; Lozoff, Brittenham, & Wolf, Reference Lozoff, Brittenham and Wolf1987), malnutrition (Galler, Ramsey, Solimano, Kucharski, & Harrison, Reference Galler, Ramsey, Solimano, Kucharski and Harrison1984; Grantham-McGregor, Powell, Walker, Chang, & Fletcher, Reference Grantham-McGregor, Powell, Walker, Chang and Fletcher1994), and inadequate social stimulation (Bradley et al., Reference Bradley, Cladwell, Rock, Ramey, Barnard, Gray and Johnson1989; Grantham-McGregor, Powell, Walker, & Himes, Reference Grantham-McGregor, Powell, Walker and Himes1991; Ramey & Ramey, Reference Ramey and Ramey1998).
Measuring executive function in young children in resource-limited settings is difficult. Measures that take into account immature verbal and motor skills and low attentional spans, and adapted to local conditions, are required (Espy, Reference Espy2004). One task designed to test motor inhibition that has been translated and adapted for various cultures is the A-not-B task. In this test, an object is hidden repeatedly at location A and then at location B. Infants who persistently search at A show poor motor inhibition. Devised by Piaget (Reference Piaget1954) to study object permanence in infants, initial cognitive explanations regarding this task focused on short term memory (Schacter, Moscovitch, Tulving, McLachlan, & Freedman, Reference Schacter, Moscovitch, Tulving, McLachlan and Freedman1986). Subsequently, it was suggested that executive functions were important (Diamond, Reference Diamond1988; Diamond & Goldman-Rakic, Reference Diamond and Goldman-Rakic1989): two meta-analyses of over 100 studies (Marcovitch & Zelazo, Reference Marcovitch and Zelazo1999; Wellman, Cross, & Bartsch, Reference Wellman, Cross and Bartsch1986) both proposed that the infant's response is driven by interaction between representation of the location and inhibition of the motoric tendency to search at the previous location. Initial findings suggested that younger infants perseverate more than older infants. Subsequent longitudinal studies (e.g., Clearfield et al., Reference Clearfield, Diedrich, Smith and Thelen2006) showed that at first infants do not perseverate, that this is followed by a stage of perseveration and then at around 12 months competence on the task. Thus Clearfield and colleagues argue that performance on A-not-B may be determined by competition between active (faster) and latent (slower) memory processes, and perseveration may be a consequence of immaturity in processes that guide application of past experience. Initially, Thelen and Smith (Reference Thelen and Smith1994) attributed perseveration to motoric rather than cognitive schemes, but more recently Smith, Thelen, Titzer, and McLin (Reference Smith, Thelen, Titzer and McLin1999) proposed that infants’ reaching is guided by a dynamic interplay between cognitive, motor, and visuospatial systems. While processes that underlie the A-not-B task are yet to be ascertained, the task covers a wide age-group, is sensitive for even very young infants (e.g., Clearfield et al., Reference Clearfield, Diedrich, Smith and Thelen2006; Diamond & Doar, Reference Diamond and Doar1989) and, hence, seems suitable for measuring early executive function.
A second important aspect of executive function is delay inhibition. Conflict inhibition tasks such as A-not-B require inhibiting an inappropriate prepotent response while activating a conflicting novel response. Delay inhibition tasks (e.g., the Self Control task) require the child simply to refrain from responding until a given signal, and may be purer measures of inhibition (Carlson, Davis, & Leach, Reference Carlson, Davis and Leach2005).
Executive function emerges in infancy (Diamond, Reference Diamond1985; Thompson & Nelson, Reference Thompson and Nelson2001), and is thought to integrate input from several developing systems including attention (Rothbart & Ahadi, Reference Rothbart and Ahadi1994), memory and language (Kopp, Reference Kopp1982), and psychosocial functioning (Londerville & Main, Reference Londerville and Main1981; Stayton, Hogan, & Ainsworth, Reference Stayton, Hogan and Ainsworth1971). Psychomotor skills, language and socioemotional skills develop concurrently, but draw less upon executive mechanisms and may therefore be less affected by maternal worm infections.
We aimed to examine effects of maternal worm infections and anthelminthic treatment in pregnancy on motor and cognitive outcomes in infants at age 15 months. Our hypothesis was that two measures known to have high executive loads (the A-not-B and Delay Inhibition tasks) would be particularly sensitive to effects of maternal worm infections. In a large sample, we therefore examined different subtests of a locally appropriate assessment battery, the Kilifi Developmental Inventory-KDI (Abubakar, Holding, Van Baar, Newton, & Van de Vijver, 2008), to establish effects of maternal worm infections and their treatment on infants’ developing executive functions, and other developing cognitive and psychomotor skills.
Methods
Design and Participants
This research was part of the Entebbe Mother and Baby Study (EMaBS), a double-blind randomized placebo-controlled trial of anthelminthic treatment during pregnancy (Elliott et al., Reference Elliott, Kizza, Quingley, Ndibazza, Nampijja, Muhangi and Whitworth2007). Between 2003 and 2005, a total of 2507 pregnant women were enrolled, investigated for parasitic infections and hemoglobin level, and randomized to receive albendazole (400 mg) or its matching placebo and praziquantel (40 mg/kg) or its matching placebo in a 2 × 2 factorial design. Albendazole treats roundworms (nematodes), and has some antiprotozoal effects. Praziquantel targets flatworms (trematodes), including schistosomiasis. Neither drug has known effects on bacterial infections. All mothers including those who had received placebos during pregnancy were treated effectively soon after delivery.
Infants underwent psychomotor assessments at 15 months using a modification of the KDI. Cognitive and social abilities were assessed using additional measures. Of 2507 women enrolled, there were 2345 live births of whom 1022 children were assessed but 39 were excluded from analysis because they were not tested within 2 months of age 15 months. Of those not tested, 94 had died (complications of labor or neonatal sepsis) before reaching age 15 months (infant mortality was not related to maternal worm infections [unpublished data] or their treatment [Webb et al., Reference Webb, Mawa, Ndibazza, Kizito, Namatovu, Kyosimire and Ameke2011]), 174 were lost to follow-up, 427 were seen at 15 months before the developmental assessments were introduced, 628 missed the 15 month visit. Of the 983 children (500 boys) tested, mean age was 15.59 months (SD 0.49; min 14.23 months; max 17 months). Of their mothers, 249 received praziquantel and albendazole; 241 praziquantel and albendazole-matching placebo; 251 albendazole and praziquantel-matching placebo; and 242 received both placebos.
Ethics
This research was approved by the Science and Ethics Committee of the Uganda Virus Research Institute and the Uganda National Council of Science and Technology. Written informed consent was obtained from all eligible participants. In 1996, WHO recommended mass treatment of pregnant women in the second and third trimester with albendazole (400 mg) (WHO, 1996) in settings with high prevalence of hookworm and anemia. In 2002, WHO recommended treatment of schistosomiasis with praziquantel (40 mg/kg) during pregnancy and breast-feeding (WHO, 2002) but there was limited evidence on the risk-benefit ratio of this intervention. There was concern that anthelminthic drugs might lead to adverse birth outcomes (Bradley & Horton, Reference Bradley and Horton2001) and that among HIV positive women anthelminthic treatment might lead to an increase in HIV load and increased vertical HIV transmission (Elliott et al., Reference Elliott, Mawa, Joseph, Namujju, Kizza, Nakiyingi and Whitworth2003). Thus a condition of equipoise was considered to exist (Elliott et al., Reference Elliott, Kizza, Quingley, Ndibazza, Nampijja, Muhangi and Whitworth2007). Indeed, WHO subsequently called for placebo-controlled trials of praziquantel during pregnancy (WHO, 2006). Our population had a moderate prevalence of hookworm (45%) and schistosomiasis (18%) but rates of anemia were relatively low and women with severe anemia (hemoglobin level less than 8.0 g/dL) were excluded from the study and treated. Hence a placebo-controlled study design was considered justified.
Motor and Cognitive Assessments
We used two executive function measures, the A-not-B task and a Self Control (delay inhibition) task, previously translated and used in rural Kenya (Abubakar, Holding, Van de Vijver, Bomu, & Van Baar, Reference Abubakar, Holding, Van de Vijver, Bomu and Van Baar2010). Infants’ skills on Language, Self Care and Recognition of Self and Others were determined using parental reports (Abubakar et al., Reference Abubakar, Holding, Van de Vijver, Bomu and Van Baar2010). Fine-motor and gross-motor function, and non-task behavior (mood, interaction, and activity) were assessed using ratings originally developed for the KDI (Abubakar et al., Reference Abubakar, Holding, Van Baar, Newton and Van de Vijver2008). Details of measures are described below.
Fine Motor
Control of small hand-movements was assessed using items such as building a tower with blocks and scribbling with a pen. Twenty-seven items were scored as pass/fail and summed to give a score.
Gross Motor
Control of the limbs was assessed using 35 items such as kicking a ball or climbing onto a platform; a total was calculated.
Child's mood, activity, and interaction
The assessor observed the child's activity, mood, and level of interaction and rated them on a six-point scale. High scores indicated good mood, activity, or interaction.
Self Control (delay inhibition)
In two trials, a biscuit (trial 1) or wrapped gift (trial 2) was presented to the child who was instructed not to take it until the assessor had completed what he/she was doing. Waiting time (in seconds) was recorded to a maximum of 150 s. Average waiting time for the two trials was computed.
The A-not-B task
A biscuit was placed in one of two wells as the child watched and both wells were then covered with opaque cups. The board was taken out of sight for 10 s during which the child was distracted with a song. The board was then brought back and the child asked to point to the well with the biscuit. The child was given the biscuit if she or he successfully located it. The location of the biscuit was switched to the other well after two consecutive correct responses. Ten trials were given; the number of correct responses was scored.
Language
This was assessed by interviewing the mother or guardian, asking whether the infant produced common pre-speech items such as vowels (e.g., aa, aa), babble (e.g., ma, ma) or gestures (e.g., waving for “bye’), spoke definite words, or named and identified common household objects (11 items overall).
Recognition of Self and Others
The caregiver was asked whether the infant reacted to his/her name, or distinguished his mother and other familiar people from strangers (altogether 12 items).
Self Care
The interview collected information about behaviors such as how much the infant helped during dressing and feeding (15 items).
Piloting, training, and scale reliability
The measures were translated into Luganda and piloted on 50 children. Seven nurses and two doctors were trained. To assess reliability, every 10th infant was rated by two assessors on all measures. Comparisons of 80 paired records showed inter-rater reliability coefficients ranging from 0.75 to 1.00 (Table 1). Scores showed normal distributions on all measures except Self Control. Overall psychomotor performance (mean 29.58; SD 3.79) was found to be similar to that of an age-matched sample of Kenyan infants (Abubakar et al., Reference Abubakar, Holding, Van Baar, Newton and Van de Vijver2008). Internal consistency was examined using Cronbach's alpha and poorly correlated items were deleted. The edited measures had internal consistency coefficients ranging from 0.62 to 0.78 (Table 1). Principal component analysis confirmed that motor measures loaded on one component, cognitive measures on another. There was a negative correlation (r = −0.04; p = .26) between the two measures of executive function (A-not-B and Self Control) which was not statistically significant. Psychomotor items in the KDI had exhibited high test–retest reliability in the Kenyan sample suggesting that the tool was stable over time (Abubakar et al., Reference Abubakar, Holding, Van Baar, Newton and Van de Vijver2008). These psychometric features are summarized in Table 1.
Table 1 Inter-rater reliability and internal consistency of the measures and factor loadings

Testing Procedure
Testing at age 15 months was postponed if mother or clinician judged the child to be unwell. Sessions lasted 45 to 65 min. Short breaks were allowed as judged by the assessor and mother. After the session, a small age-appropriate gift was given to the child and transport money reimbursed to the parent.
Additional Data
Parasitology
Stool samples of the pregnant women and infants were examined for helminth ova using Kato Katz technique (Katz, Chaves, & Pellegrino, Reference Katz, Chaves and Pellegrino1972), and cultured for Strongyloides stercoralis. Blood was examined for Mansonella perstans using the modified Knott's method (Melrose, Turner, Pisters, & Turner, Reference Melrose, Turner, Pisters and Turner2000) and for malaria parasites using thick smears.
Antenatal history and delivery data
We recorded information on maternal illnesses, medications and vaccines during pregnancy, mode of delivery, Apgar score at 10 min, birth weight, congenital abnormalities, and immunization received at birth.
HIV status of the child
Overall, 97 mothers tested HIV positive during pregnancy. At 6 weeks of age, their infants were tested for HIV using DNA and RNA polymerase chain reaction. Specific management of HIV positive women and exposed children is elaborated elsewhere (Elliott et al., Reference Elliott, Kizza, Quingley, Ndibazza, Nampijja, Muhangi and Whitworth2007; Mpairwe et al., Reference Mpairwe, Muhangi, Namujju, Kisitu, Tumusiime, Muwanga and Elliott2005).
Growth monitoring
Infants’ weight, height, head circumference, and mid upper arm circumference were recorded at week 6, 10, and 14, and at 6, 9, and 12 months. At 1 year, hemoglobin levels were measured.
Illnesses in infancy
Numbers of episodes of malaria, diarrhea, lower respiratory tract infections, and upper respiratory tract infections were recorded at the study clinic.
Sociodemographic data
Sociodemographic data including marital status, gravidity, age, education, occupation, income, and number of people in the home, were collected at enrolment, by interview. Household socioeconomic status was derived from items owned, building material of the house and number of rooms.
Results
Participants’ Characteristics
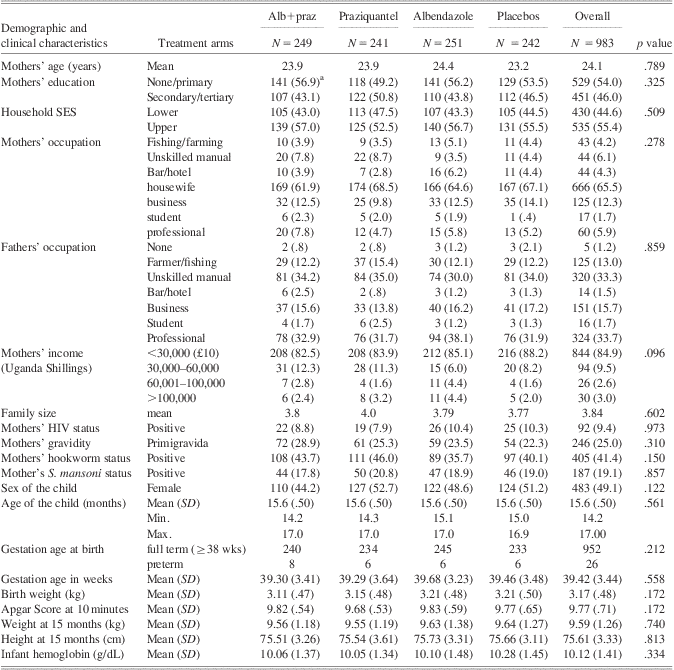
Characteristics of the 983 participating mothers and children are summarized in Table 2. Characteristics of mothers of participating and non-participating children were similar with respect to education, household socioeconomic status, and prevalence of Schistosoma mansoni infection. However, mothers of participants were older and less likely to be primigravida, and had a lower prevalence of HIV and hookworm (p < .01). Children who participated had mean birth weight, height and weight for age, and hemoglobin level within WHO normal ranges.
Table 2 Characteristics of participating women and infants

aFigures are proportions (percent) unless otherwise indicated.
Descriptive Statistics for Psychomotor and Cognitive Measures
Descriptive statistics for the various measures are summarized in Table 3. The numbers of infants who completed the tasks varied. Fewer completed the A-not-B task because they cried for the treat on the first trial and refused to continue. Scores on five of the seven measures were normally distributed. Performance on Self Control was slightly positively skewed and Recognition of Self and Others was negatively skewed. Appropriate transformations were conducted before analysis.
Table 3 Descriptive statistics of infants’ scores on the various measures

Note. The numbers under each domain are discrete scores therefore no units are available.
Prevalence of Worms in Mothers
Prevalence of worm species identified in the mothers is shown in Table 4. Hookworm had the highest prevalence followed by Mansonella perstans. Thirty percent of mothers had mixed infections with some women having up to five species of worms. A total of 352 (34.9%) women did not have any worm. Only effects of maternal worms with at least 2% prevalence were subsequently analyzed.
Table 4 Prevalence of the various worms in the pregnant women whose infants were assessed on the motor and cognitive measures at 15 months

Effects of Albendazole and Praziquantel
T tests were used to compare performance between children born to treated versus untreated mothers first across the whole sample to assess the benefit of mass treatment, and then just those where the mothers had worms susceptible to the drugs. We found no interactions between albendazole and praziquantel (p > .05). Therefore, the effect of each drug was examined separately (albendazole vs. placebo; praziquantel vs. placebo). Overall neither albendazole nor praziquantel had significant effects on developmental outcomes (all values of p > .05). Of note, after delivery stool analysis showed a significant decline of the prevalence of hookworm and schistosomiasis among albendazole or praziquantel-treated women, respectively, but no reductions in Mansonella perstans, Trichuris trichiura, and Strongyloides stercoralis (Ndibazza et al., Reference Ndibazza, Muhangi, Akishule, Kiggundu, Ameke, Oweka and Elliott2010).
We found, however, a significant effect of praziquantel on the A-not-B task and Self Control in children whose mothers were infected with schistosomiasis during pregnancy. Children whose mothers were treated for schistosomiasis during pregnancy performed better on Self Control than those who were not treated [t(140) = 2.58; mean difference = 5.96; p = .01; Cohen's d = 0.47]. However for the A-not-B task, children whose mothers were treated performed more poorly than those whose mothers were not treated [t(145) = −2.23; mean difference = −0.93; p = .03; Cohen's d = .37]. We found no effect of albendazole among infants of mothers with the principal susceptible species, hookworm.
Associations Between Maternal Worm Infections And Other Exposures, And Infant Outcomes
Raw Correlations
First, raw correlations between child test scores and exposure variables were explored to identify factors that might mediate or mask effects of maternal worm infections. Maternal worms included were Schistosoma mansoni, hookworm, Mansonella perstans, Ascaris lumbricoides, Trichuris trichiura, and Strongyloides stercoralis.
Background factors included were mother's age, education, occupation, income, gravidity, HIV status, and hemoglobin at enrolment; household socioeconomic status, family size and father's occupation; and the child's sex, gestational age at birth, birth weight, Apgar score at 10 min, malaria, diarrhea, and respiratory infections in the first year, activity, mood and interaction level during assessment, hemoglobin, weight, and height. These correlations and regressions were examined across the entire sample irrespective of treatment status since neither treatment showed any overall effect.
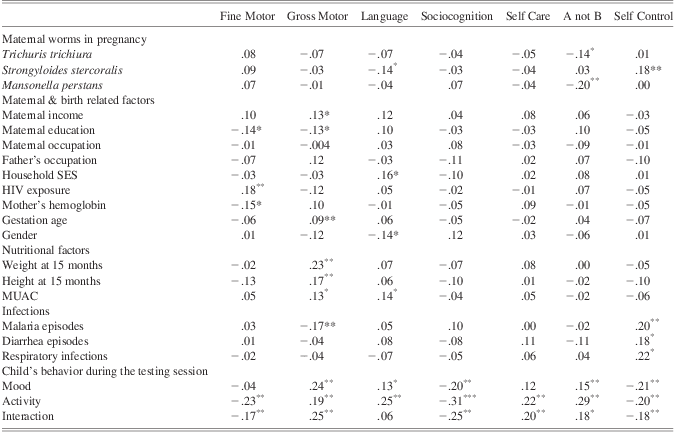
Three maternal worms showed correlations with the infant test scores: Strongyloides stercoralis correlated positively (in the opposite to expected direction) with scores on Self Control and negatively with Language. Trichuris trichiura and Mansonella perstans correlated negatively with scores on the A-not-B task. Other factors variably correlated with performance on the motor and cognitive measures. Of 112 correlations calculated, 34 were significant at the 0.05 cutoff, even after adjusting for multiple comparisons with false discovery rate. Only exposures with at least one significant correlation with the outcomes are shown in Table 5.
Table 5 Pearson correlations between maternal worms and other health and sociodemographic exposures, and the outcomes

Note. MUAC = mid upper-arm circumference.
*p < .05.
**p < .01.
***p < .001.
Multivariate Regression Analysis
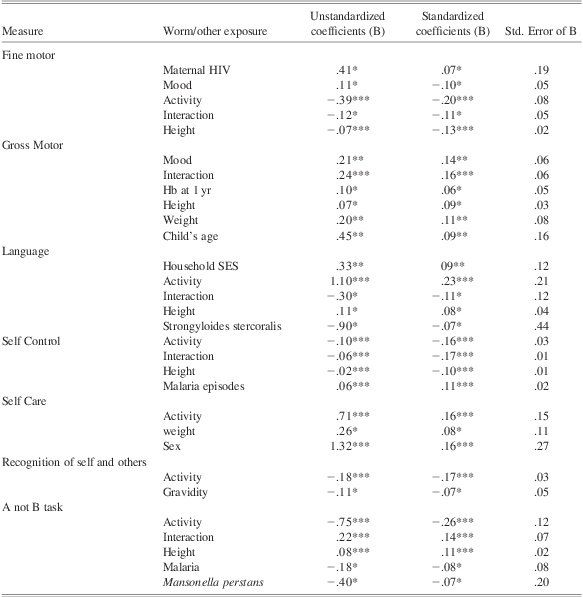
To test the hypothesis that maternal worm infections would independently affect performance of children on measures of executive function, we performed a series of hierarchical linear regression analyses in which associations with maternal worms were examined taking into account associations with potential confounding factors. Using this technique we explored the unique contribution of maternal worm infections over and above effects of additional exposures. The independent variables were entered in a single model which comprised three blocks: maternal-related factors (SES and family factors, health factors), child-related factors (gender, health, and behavioral factors), and maternal worms (entered in that order). Each subtest of the motor and cognitive assessment was examined separately. Categorical measures (worm exposure and drug treatment) were loaded as dummy variables. Non-significant explanatory variables were dropped from the model one at a time leaving only the significant factors and therefore the best model that accounts for performance on each of the measures. Associations between maternal worm infections, and other factors, and each of the outcomes are summarized in Table 6 and described below; the order of entering variables into the analysis is also listed in this table.
Table 6 Effects of maternal worm infections and of other health and sociodemographic exposures on the various functions

Note. Maternal-related factors were entered into the model in this order: mother's age, education level, occupation, income, gravidity, HIV status, and hemoglobin level at enrollment; household socioeconomic status, family size, and father's occupation. Child factors were entered into the model in this order: child's sex, gestation age at birth, birth weight, Apgar score, malaria, diarrhea, and respiratory infections in the first year, activity, mood, and interaction level during assessment, hemoglobin level, weight, and height. Hb = hemoglobin; SES = socioeconomic status.
*p < .05.
**p < .01.
***p < .001.
Fine motor
Child's mood and maternal HIV status were positively associated with fine motor function, whereas activity, interaction and height were negatively associated with this outcome (R 2 = 0.08; F(5,956) = 15.89; p < .001). Maternal worm infections had no association.
Gross Motor
Performance on Gross Motor was positively associated with the child's hemoglobin level at 1 year, height and weight at 15 months, mood and interaction during assessment and child's age (R 2 = 0.13; F(6,884) = 21.76; p < .001). Maternal worms had no association.
Language
Maternal Strongyloides stercoralis showed a negative association with infants’ Language. Household socioeconomic status, child's activity and height at the time of assessment showed positive associations with Language; and child's interaction during assessment showed a negative association (R 2 = 0.06; F(6,860) = 9.42; p < .001). This model accounted for more variance than the model without maternal worms (ΔR 2 = 0.005).
Self Care
This measure was positively associated with child's sex, activity, and weight at the time of assessment (R 2 = 0.05; F(3,974) = 16.90; p < .001). Maternal worms had no association.
Recognition of Self and of Others was negatively associated with maternal gravidity and activity of child during assessment (R 2 = 0.03; F(2,975) = 16.29; p < .001). Maternal worm infections had no association.
Self Control
Performance on Self Control was negatively associated with infant's height, activity, and interaction during assessment and positively related with malaria episodes in infancy. The raw association with maternal Strongyloides stercoralis infection was no longer significantly associated with the function when we controlled for the other exposures (R 2 = 0.11; F(5,810) = 19.94; p < .001).
A-not-B task
Child's activity, interaction, and height were positively associated, while malaria episodes in infancy and maternal Mansonella perstans were negatively associated on this outcome. The raw association with maternal Trichuris trichiura was no longer significant when we controlled for other factors (R 2 = 0.16; F(5,774) = 28.71; p < .001). The model with Mansonella perstans infection accounted for more variance than the model without the worm (ΔR 2 = 0.004).
In summary, significant maternal worm effects were observed between Mansonella perstans and performance on the A-not-B task, and between Strongyloides stercoralis and performance on Language. Hookworm and schistosomiasis did not show significant associations with the outcomes although these were the most prevalent worms in the sample.
Discussion
The Impact of Anthelminthic Treatment During Pregnancy
Treating pregnant women with albendazole or praziquantel had no significant effects on infant developmental measures. The absence of treatment effects in a setting with high prevalence of worm infection suggests that there is not much benefit of single-dose mass anthelminthic treatment during pregnancy for cognitive and psychomotor outcomes in the child.
Subgroup analysis suggested that praziquantel treatment in mothers who had Schistosoma mansoni infection had significant effects on both the A-not-B and Self Control tasks even though the worm did not have significant effects on these outcomes. We observed a positive effect on Self Control. However, the negative effect of the drug on the A-not-B task is difficult to explain since Schistosoma mansoni infection has been associated with cognitive deficits and treatment was therefore expected to be beneficial. This result could have occurred by chance, or the negative effect of praziquantel might be linked to changes in the host immune responses following treatment. Treatment of schistosomiasis is associated with an increase in circulating proinflammatory as well as anti-inflammatory cytokines (Azim, Sedky, el-Tahawy, Fikry, & Mostafa, Reference Azim, Sedky, el-Tahawy, Fikry and Mostafa1995; Mwatha et al., Reference Mwatha, Kimani, Kamau, Mbugua, Ouma, Mumo and Dunne1998); the killing of schistosomes following praziquantel treatment results in release of worm antigens and a boost in anti-worm responses (Tweyongyere et al., Reference Tweyongyere, Mawa, Ngom-Wegi, Ndibazza, Duong, Vennervald and Elliott2008; Walter et al., Reference Walter, Fulford, McBeath, Joseph, Jones, Kariuki and Dunne2006). The infection may be effectively cleared but the high concentration of cytokines induced might perhaps interfere with neurotransmitter systems, particularly the dopaminergic systems, thereby affecting cognitive functioning (Reichenberg et al., Reference Reichenberg, Yirmiya, Schuld, Kraus, Haack, Morag and Pollmacher2001).
Effects of Maternal Worm Infections
The results provide some support for the main hypothesis that maternal worm infections in pregnancy might have negative effects, particularly on infant executive function, but this is restricted to only two species. In line with our prediction, certain maternal worm infections were associated with performance on both the A-not-B and Self Control tasks. We observed a negative association between maternal Mansonella perstans and infants’ later performance on the A-not-B task and a positive correlation between Strongyloides stercoralis and Self Control. The latter was contrary to our predictions and might be an incidental finding, as it suggests that infection improves infant's skills on this function. It could be that children born to mothers with this infection are not more self-controlled but rather less active and less interested in their environment, and hence less interested in the treat. Indeed infant malaria and respiratory infections likewise showed positive associations with performance on this measure. Thus apathy may mediate the effect of ill-health on Self Control. However, delay tasks may not be reliable measures of inhibitory control since performance on these measures varies greatly with the task, including the type of treat used (Carlson et al., Reference Carlson, Davis and Leach2005).
The results suggest that executive functions might be particularly susceptible to influences of maternal worms; the effect observed on language has been reported in earlier childhood studies of worms (e.g., Ezeamama et al., Reference Ezeamama, Friedman, Acosta, Bellinger, Langdon, Manalo and McGavey2005), and this might be secondary to effects on executive function given that the two domains emerge within the same period and are reported to be interdependent throughout life. Moreover, the two domains often show comorbidity (Ribeiro et al., 2011; Tannock & Schachar, 1996; Willinger et al., 2003 for a review). Rebiero et al. explored the comorbidity between language and executive functions and found that early executive function (attention) impairment predicted later language problems but not vice-versa. Therefore, the disruption of executive function by worms may explain the effect observed on language. Given these specific influences, the results suggest that maternal worm infections during pregnancy do not cause generalized cognitive deficits in infancy. Plausible explanations for the selective nature of maternal worm effects in infancy have been proposed. Maternal worms are believed to compete with the fetus for nutrients that are vital for formation of neurological systems (Beard et al., Reference Beard, Unger, Bianco, Paul, Rundle and Jones2007).
Naismith (Reference Naismith1969) suggests that, in cases of moderate deprivation (e.g., due to mild worm infections), the fetus takes priority over the nutrients that remain and thrives with minimal effects. Executive functions may however be more vulnerable due to more specific metabolic effects. It has been proposed, for example, that the availability of certain neurotransmitters is dependent on the dietary supply of their amino acid precursors (Wainwright & Colombo, Reference Wainwright and Colombo2006); for example, tryptophan is the dietary precursor of serotonin, and tyrosine is the precursor for dopamine and norepinephrine (Fernstrom, Reference Fernstrom1990). Reduced levels of tyrosine may lead to impaired executive function in children with phenylketonuria (Sharman, Sullivan, Young, & McGill, Reference Sharman, Sullivan, Young and McGill2009). Therefore, changes in the availability of different amino acids may result in disturbances of specific brain functions and behavior. Dopamine, in particular, acts in the prefrontal cortex in which executive functions including planning, inhibition, and attention are represented, both in animals (Gaarlen, Brueggeman, Bronius, Schoffelmeer, & Vanderschuren, Reference Gaarlen, Brueggeman, Bronius, Schoffelmeer and Vanderschuren2006) and humans (Goto & Grace, Reference Goto and Grace2005; Smith & Kieval, Reference Smith and Kieval2000).
However, in worms as in other organisms, tyrosine is an essential nutrient (Moran, Reference Moran2005). Impaired tyrosine catabolism in worms is associated with dramatic effects including a short life span, destruction of the intestine, and decreased fertility. Given that intestinal worms depend on the host's digestive contents for this amino acid, prolonged infestation of worms may deprive the body of this nutrient and this may in turn impact on dopamine synthesis resulting in impairment of the executive function.
The associations observed in this study were based on only two measures of executive function. It is possible that worms affect other executive skills not measured by the Delay inhibition task, and by the A not B task. Moreover, because assessments were done later in infancy, we may not completely rule out the possibility that earlier in life worms could show more generalized cognitive deficits. These are important issues that need to be resolved by longitudinal studies measuring various cognitive abilities.
Effects of maternal worm infections on infant cognitive functioning could also be mediated by iron deficiency. However, in this study we found very weak associations between maternal worm infections and maternal anemia (Muhangi et al., Reference Muhangi, Woodburn, Omara, Omoding, Kizito, Mpairwe and Elliot2007), or benefit of anthelminthic treatment for anemia (Ndibazza et al., Reference Ndibazza, Muhangi, Akishule, Kiggundu, Ameke, Oweka and Elliott2010). Moreover, all women were routinely treated with iron and folic acid during pregnancy, perhaps compensating for any iron loss due to worm infections. Worms were not causing major iron deficiency anemia in these mothers, so cognitive effects of worms mediated by maternal anemia would not have been detectable in this study.
In addition to worm infections, infant abilities were examined in relation to other factors. Common infections in infancy, particularly repeated malaria, were found to influence infants’ performance. As expected, child nutritional factors (hemoglobin, height, and weight) showed significant positive correlations with developmental outcomes. Infant mood, activity, and interaction level also consistently influenced scores on the various developmental measures highlighting the role non-task behavior plays in performance. Given that numerous factors were affecting infants’ development before and after birth, a relatively small amount of variance explained by worms alone might be expected. However, worms affect millions of mothers and children, and hence, the global impact of even a small effect on child development may be important enough to raise concern. Regardless of the effect size, these findings are in keeping with our prediction and deserve further exploration.
Our study had an experimental design, and this was a strength of our analysis of the effects of anthelminthic intervention. However, it is of note that the majority of pregnant women who participated had a low intensity of worm infections; mothers with hemoglobin below 8 g/dL were excluded from the study, and iron and folic acid were given during antenatal care. These characteristics limit the extrapolation of our findings to other populations. It could be that, where infections are heavy and nutritional and micronutrient status is marginal, maternal worms have stronger and more diverse effects on the development of the executive system. Future studies should aim specifically to investigate effects in populations with high intensities of worms.
Furthermore, in early infancy, cognitive processes are not yet fully developed, which undermines the validity of developmental effects measured. Moreover, future studies should assess effects of worms on other aspects of executive functions, other than those measured in this study. We continue to follow-up these infants and reassess the worm effects at older ages and to include aspects of executive functions not examined in this study to re-evaluate the selectivity of these effects.
In conclusion, the results of this study suggest that certain maternal worm infections during pregnancy may have negative influences on early executive function in the offspring but anthelminthic treatment is unlikely to reverse these effects. Further research should aim to replicate these findings in the light of the limitations mentioned above.
Acknowledgments
This research was funded by Wellcome Trust grant numbers 064693 and 079110 as part of the overall Entebbe Mother and Baby Study. We thank the EMaBS team for their hard work and commitment. We also thank the team in Kenya Medical Research Institute for permission to use their measures and support in training the assessors. We thank the mothers and children from Entebbe and Katabi for their participation in the study. There were no conflicts of interest in this study.