INTRODUCTION
Acute radiotherapy (RT) side effects involving the skin include pain, discomfort, irritation, itching and burning.Reference McQuestion 1 Typical skin reactions begin with erythema and may progress through stages of dry desquamation, moist desquamation and ulceration.Reference McQuestion 1 – Reference Salvo, Barnes and van Draanen 3 Skin reactions are patient specific and factors affecting the onset and severity of skin reactions are treatment and patient dependent. Treatment associated factors include target location, beam type, energy and technique, fraction size, fraction and total dose, and use of dose modifying materials such as bolus, wedges and filters. Patient related factors may include age, concurrent therapy (e.g., chemotherapy) and condition of the skin which may be affected by previous surgery or disease, patient’s typical skin routine and previous sun exposure.Reference McQuestion 1 , Reference Salvo, Barnes and van Draanen 3
Oncology staff provide information and interventions to manage these acute effects. Information includes instructions on maintaining skin cleanliness and integrity preventatively. Interventions may include medicated topical agents and dressings to manage discomfort, avoid infection and promote healing.Reference McQuestion 1 , Reference D’Haese, Van Roy, Bate, Bijdekerke and Vinh-Hung 4 , Reference Morley, Cashell, Sperduti, McQuestion and Chow 5
Optimal prevention and management of skin reactions in RT is complicated by wide-spread concerns among professionals in the field that applying products on the skin during radiation delivery will increase skin dose and toxicity.Reference Bieck and Phillips 6 – Reference Burch, Parker, Vann and Arazie 8 Patient education often includes teaching patients to avoid applying creams, lotions and deodorants on the skin before RT due to fear of increasing the severity of skin reactions.Reference Bolderston, Lloyd, Wong, Holden and Robb-Blenderman 2 , Reference Harper, Franklin, Jenrette and Aguero 9 Informal surveys in our department revealed that staff members routinely instruct patients to avoid applying any skin products 1–2 hours before treatment or to wipe off residual creams before treatment delivery, in spite of any institutional practice guidelines recommending these restrictions. The use of dressings or other topical products in wound care by nursing staff was also restricted during the course of treatment. Staff indicated this was a common practice learned primarily from colleagues although it was not known whether or not this was evidence based. One studyReference Bieck and Phillips 6 discovered that some American and Canadian cancer centres provide no restrictions to patients applying topical agents to the skin, while others gave specific time restrictions and some centres instructed patients to completely avoid applying topical agents to the skin at any time before RT. Only one institution was found to reference their practice using evidence based guidelines.Reference Morley, Cashell, Sperduti, McQuestion and Chow 5
Variations in skin care management instructions can be distressing and confusing to patients, especially if the information is conflicting or complicated.Reference Bolderston, Lloyd, Wong, Holden and Robb-Blenderman 2 , Reference Morley, Cashell, Sperduti, McQuestion and Chow 5 , Reference Aistars 7 Instructions for applying moisturiser regularly combined with restrictions based on complicated radiation physics principles may not be easy for patients to interpret. Consultation with patients in our institution found that patients may alter their skin care regimen in unintended ways in the face of this complicated and apparently conflicting information. Patients are less confused and have better self-care adherence when provided with consistent and straightforward care instructions.Reference Mannix, Bartholomay, Doherty, Lewis and Bilodeau 10
Restrictions in the management of skin toxicity appear to be wide-spread and primarily based on two main concerns:Reference Fackrell, Kirby, Sanghera and Hartley 11
-
(1) An increase of dose due to higher photon interactions from the metallic component of the creams.
-
(2) A bolus effect created by an increased layer added to the surface of the skin, leading to an increased skin reaction.
The effect on skin dose of moisturisers and other commonly used products has been reported in several studies. One such studyReference Morley, Cashell, Sperduti, McQuestion and Chow 5 determined that a clinically meaningful increase in skin dose required at least 0·7 mm of product on the skin during treatment delivery under the least favourable conditions tested. A realistic application of such products is closer to 0·3 mm.Reference Rietschel 12 Skin care instructions and wound management practices were simplified and less restricted in our institution following these results.Reference Morley, Cashell, Sperduti, McQuestion and Chow 5 An early but sentinel study by Burch et al.Reference Burch, Parker, Vann and Arazie 8 investigated the surface dose on 15 different products using a Markus type parallel plate ionisation chamber in a polystyrene phantom (six deodorants, two powders and seven lotions) and found no large increase in surface dose. The study did not specify which products were tested, but indicated that the lotions were safe for use during RT from a dose to skin perspective.Reference Burch, Parker, Vann and Arazie 8
Dressings are potentially much thicker and variable in density and material components compared with oil or water-based creams. A survey was done with 18 oncology centres in CanadaReference Thomas, Reimer-Kirkham and Kohr 13 on their policy of removing wound dressings during RT. Results showed that half of the surveyed centres would leave the dressings in place during treatment, while the remaining centres would remove the dressings. These decisions were primarily based on individual evaluation and personal practices rather than department policies or evidence.
There are inconsistencies reported in the literature with respect to the standard of care of when to use topical agents and dressings during RT. Several studies measure dose under such products, however interpreting the results of these studies requires consideration of the different approaches to measuring skin dose, the different products tested, and the different beam parameters used. Measurement of superficial dose can be challenging and may be approached using a variety of instruments and at varying depths to approximate dose to skin epithelium. Several studiesReference Morley, Cashell, Sperduti, McQuestion and Chow 5 , Reference Burch, Parker, Vann and Arazie 8 , Reference Fackrell, Kirby, Sanghera and Hartley 11 , Reference Thilmann, Adamietz, Mose, Saran, Ramm and Bottcher 14 use a variety of measurement techniques at different depths. The epithelial layer varies according to properties such as location, age, skin condition and other factors.Reference Takema, Yorimoto, Kawai and Imokawa 15 In one particular study, the depth of the epidermis was measured between 64 and 123 µm,Reference Koehler, Vogel, Elsner, Konig, Buckle and Kaatz 16 and another study measured the epidermis to be 74·9–96·5 µm.Reference Sandby-Moller, Poulsen and Wulf 17 Care must be taken in interpreting and comparing such results and relating them to other relevant clinical scenarios. In practice, the most important depth for skin reactions will vary by skin type, patient factors, and area of the body under consideration. Understanding the dosimetric impact of various surface materials is improved when the dose impacts are compared to other relevant scenarios for which clinicians have an established frame of reference. The relative dose increase at other reference depths will be approximatley proportional given the shape of photon depth dose curves in the build-up region.
This study aims to build on previous workReference Morley, Cashell, Sperduti, McQuestion and Chow 5 by measuring relative skin dose enhancement for a range of products commonly used in RT. These include products potentially underutilised due to dose concerns, and products containing high atomic number materials that appear to raise concern among practitioners. Dressings in a dry and wet state will also be studied. These relative doses are compared, under the same conditions, to skin dose enhancement from relatable clinical scenarios such as under thermoplastic immobilisation material and SuperflabTM bolus; the purpose of this study is not to determine the absolute dose to the epidermis. Conclusions should inform clinical practice regarding patient education, preventative care instructions, and the management of skin changes during RT.
MATERIALS AND METHODS
Products tested
The skin products used in the study are shown in Table 1. Inclusion of products in this study is neither an endorsement nor claim of efficacy. Products were chosen because they are commonly used in RT practice, represent a general class of product commonly selected by patients based on our experience, or because they contain high atomic number components (e.g., zinc, iodine and silver). Products selected were not intended to be exhaustive, but were intended to cover a spectrum of materials covering the types of products practitioners may encounter in clinical practice.
Table 1 List of creams, lotions, and gels used in this study

Abbreviations: SBBC, silicone-based barrier cream; RT, radiotherapy; SPF, sun protection factor.
Dressings used in the study are shown in Table 2. Absorbent materials were tested under both dry and wet conditions to test situations where the dressing may be present over a dry wound or a heavily exudative wound that may soak the dressing material over time.
Table 2 List of dressings used in this study
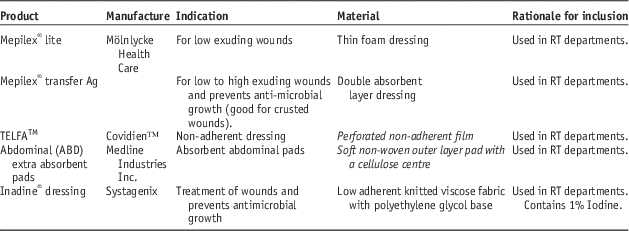
Abbreviation: RT, radiotherapy.
Beam geometery
The effect of varying photon field size and beam obliquity was previously studied.Reference Morley, Cashell, Sperduti, McQuestion and Chow 5 A single beam geometry was therefore selected for this study and can be related to other conditions tested previously.Reference Morley, Cashell, Sperduti, McQuestion and Chow 5 A photon beam energy of 6 MV was selected since it was the lowest photon beam energy readily available and lower MV energies are relatively uncommon. Higher photon beam energies are more sparing and would therefore yield less relative dose increase with products on the skin surface. Moreover, 6 MV photon beams are currently the most widely used for intensity modulated radiation therapy (IMRT) and volumetric modulated arc therapy (VMAT). A 10×10 cm2 field size was used to simulate large open fields; smaller fields with less scatter contribution would have less relative dose increase with products on the surface as shown in the previous study.Reference Morley, Cashell, Sperduti, McQuestion and Chow 5
Comparative clinical scenarios
Any additional material on the surface will produce superficial dose enhancement. The clinical relevance of such increase must be determined by comparing to other relevant clinical scenarios. Most centres consider the increased scatter dose arising from thermoplastic immobilisation material on the surface on the order of 18%Reference Lee, Chuang and Quivey 18 to 28%Reference Morley, Cashell, Sperduti, McQuestion and Chow 5 to be acceptable.Reference Velec, Waldron and O’Sullivan 19 On the other end of the spectrum, 5 mm SuperflabTM (Mick Radio-Nuclear Instruments Inc.) bolus is commonly used to intentionally increase skin dose. Measurements were therefore taken in the presence of one layer of thermoplastic mask, 5 mm SuperflabTM bolus and 10 mm SuperflabTM bolus for comparison.
Metal oxide semiconductor field effect transistor (MOSFET) measurements
Figure 1 shows the experimental setup of our dosimetric measurement. The same methodology was used as the study by Morley et al.Reference Morley, Cashell, Sperduti, McQuestion and Chow 5 A MOSFET detector (TN-1002RD; Thomson and Nielsen Electronic, Ottawa, ON, Canada) was located on top of the 5 mm SuperflabTM bolus, and was centred at the central beam axis (vertical broken line). Advantages of using a MOSFET detector include its small size, accuracy at low dose, immediate reuse and its ability to record dose history.Reference Chow and Leung 20 All MOSFET detectors used in this study were calibrated using our local standard ionisation chamber and electrometer. The density of bolus is equal to 1·02 g/cm3 and is made of tissue-equivalent SuperflabTM plastic. In Figure 1, the MOSFET detector was covered by a 1 mm polymethyl methacrylate (PMMA) layer mimicking the approximate depth of epithelium and critical dermal tissues. The most clinically relevant skin depth depends on properties such as location, age, skin condition and other factors;Reference Takema, Yorimoto, Kawai and Imokawa 15 such a specific clinical depth is not required to achieve the purposes of this study. Skin products were applied on the surface of the PMMA layer irradiated by the 6 MV photon beam, produced by the Varian TrueBEAM linear accelerator (Varian Medical Systems). A Solid Water slab (SW-457; Gammex RMI, Middleton, WI, USA) of 25×25×5 cm3 was placed underneath the bolus to provide adequate backscatter. The source-to-surface distance (SSD) was set to 100 cm at the PMMA surface and the field size of the 6 MV photon beam was equal to 10×10 cm2. The linear accelerator was calibrated to deliver 1 cGy/monitor unit (MU) at the depth of the maximum dose (1·5 cm). In this study, 100 MUs were given in each measurement with a dose rate of 600 MU/minute. Repeat measurements were obtained and averaged for each experimental condition.
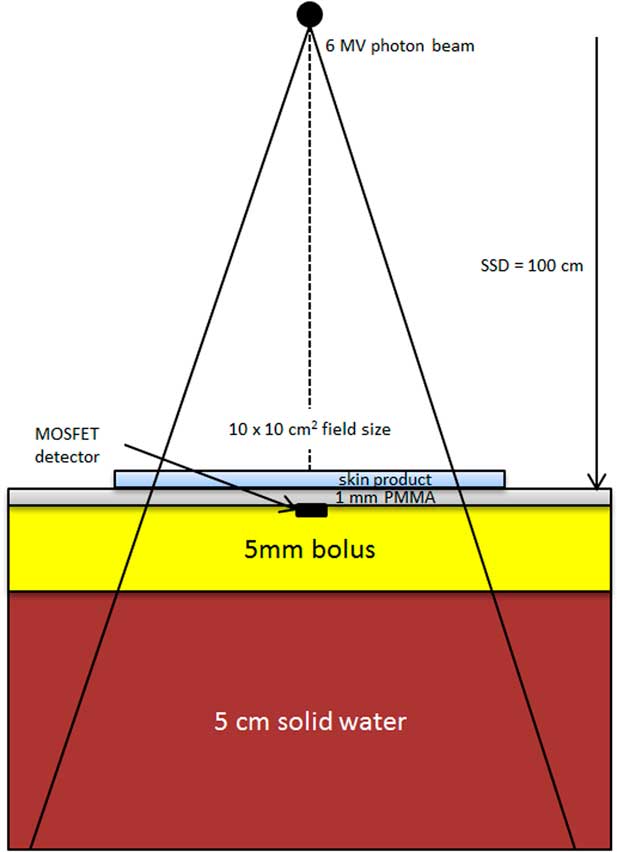
Figure 1 Schematic diagram (not to scale) showing the experimental setup of our dosimetric measurement. Abbreviation: PMMA, polymethyl methacrylate.
Experimental setup
Plastic wells with dimensions of 12×12×0·15 cm3 were created and filled with cream, lotion or gel. There was an effort to create a uniform and even layer within the well. The thickness of topical agent was noted to be much more than normally observed on a patient’s skin and thicker than a patient would likely be able to maintain for any length of time. The dose implications of thinner applications of these products were estimated to be proportional to the thickness for the tested geometry based on a previous study under the same conditions.Reference Morley, Cashell, Sperduti, McQuestion and Chow 5
Single layer dressings were laid flat, ensuring the dressing covered the 10×10 cm2 radiation field by at least 2 cm on all sides. Dressings were tested in both wet and dry states given that clinical applications may result in wound exudate accumulating in the dressings under some conditions. Wet dressings were prepared by soaking the dressings in water, laying them flat, and allowing excess water to drain off. Wet dressings were considered comparable to dressings applied to a moist wound long enough to be maximally saturated with wound exudate (including serum, fibrin and blood cells).
RESULTS
Repeatability of the MOSFET measurements was found to be the same as in previous work. The relative dose under the creams, lotions, and gels at specified thickness is shown in Figure 2. Relative dose was calculated by dividing the tested agents’ dose by the dose of the control (no tested agent).
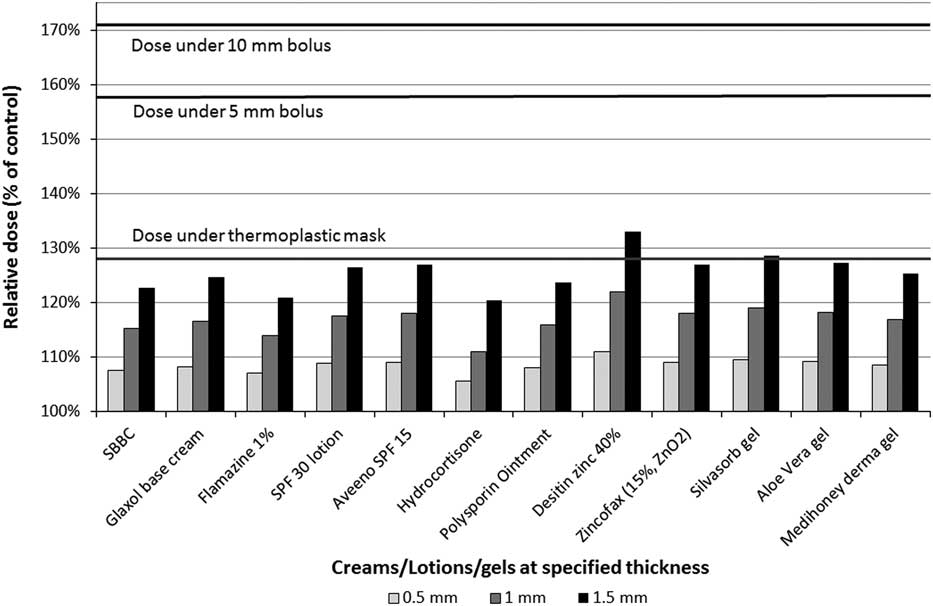
Figure 2 Relative dose under creams, lotions and gels by their thickness. All products produced much less skin dose than the thermoplastic mask for realistic application thickness (0·5 mm). Abbreviations: SBBC, silicon-based barrier cream; SPF, sun protection factor.
Relative skin dose under reference materials was 128% (thermoplastic mask), 158% (5 mm bolus) and 171% (10 mm bolus).Reference Morley, Cashell, Sperduti, McQuestion and Chow 5 At maximum tested thickness of 1·5 mm, the relative dose under all topical agents did not exceed that of thermoplastic mask with the exception of Desitin® zinc 40% (133%) and Silvasorb® gel (129%). All products produced much less skin dose than the thermoplastic mask for more realistic application thickness (0·5 mm). The relative dose versus dressings graph is shown in Figure 3. Mepilex® lite (wet) and Mepilex® Ag transfer (wet) exhibited a relative dose greater than the dose under the thermoplastic mask (133 and 141%, respectively) but not above the bolus references. All other tested dressings yielded relative doses under 111%.
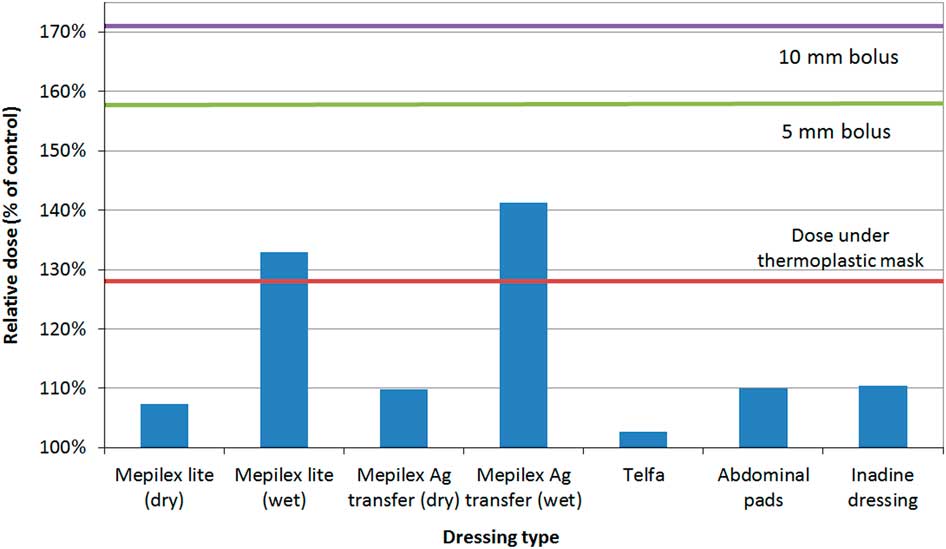
Figure 3 Relative dose under dressings. All tested dressings (except for Mepilex® lite (wet) and Mepilex® Ag transfer (wet)) yielded relative dose under 111%. Wet Mepilex® lite and wet Mepilex® Ag transfer had a relative dose greater than the dose under the thermoplastic mask (133 and 141%, respectively) but not above the bolus references.
DISCUSSION
Most centres generally consider the dose under a thermoplastic mask for treatments of head and neck cancers to be acceptable, therefore it seems reasonable to accept similar relative doses under products used to prevent or manage skin reactions from treatment. Relative doses <128% should therefore be acceptable, particularly considering that the tested products are unlikely to be present for every treatment fraction, unlike mask material or bolus. None of the tested products were close to producing dose enhancement comparable to that of 5 mm bolus.
The relative dose versus creams, lotions and gels were plotted against the thickness of the topical agents. Although the tested thickness of the topical agents was 1·5 mm, information from previous work including Monte Carlo simulation and physical measurementsReference Morley, Cashell, Sperduti, McQuestion and Chow 5 under similar conditions allowed extrapolation for 0·5 and 1 mm thicknesses.
A 1·5 mm application of cream, lotion or gel appears to be an excessive amount to expect a patient to apply; the prepared 1·5 mm samples demonstrated that this volume of product would drip down any non-horizontal surface. This thickness was used for dose measurement to assist with creating reproducible testing conditions. Other studiesReference Burch, Parker, Vann and Arazie 8 , Reference Fackrell, Kirby, Sanghera and Hartley 11 did not measure the thickness of the topical agent, but relied on applying a ‘normal application’ that a patient would routinely apply on their skin. A realistic amount of moisturiser is around 0·3 mm.Reference Rietschel 12 In Figure 2, none of the topical agents exceeded the dose under the thermoplastic mask if the thickness was 0·5 mm. Even topical agents with high atomic number constituents (Desitin® zinc 40% and Zincofax® 15%) did not have a higher relative dose compared with the thermoplastic mask at 1 mm thickness. These products are not commonly used by RT patients; their inclusion was to address concerns expressed by some clinicians for the potential bolus effect due to metallic components of topical agents.
Figure 3 represents the relative dose under various dressings. Mepilex® lite and Mepilex® Ag transfer were tested in wet and dry simulations. All dry dressings produced less dose enhancement compared with the thermoplastic mask. In real applications, it is unlikely that dressings would be maximally saturated with fluid for any significant portion of a RT treatment course. Wet dressings did produce a dose enhancement greater than the thermoplastic mask (Mepilex® Lite, 133% and Mepilex® Ag transfer, 142%) but well under the dose for 5 mm and 10 mm Superflab™ bolus. These dose enhancements are not of a concern if the dressing is partially wet, present for only a portion of RT fractions, or both. Clinical experts in this field state that daily removal of dressings before treatment is not ideal due to factors such as pain associated with the removal of the dressings and the cost of supplies associated with repeated removal of the dressings.Reference Thomas, Reimer-Kirkham and Kohr 13 In cases where RT is directed at an existing wound with large amounts of exudate or bleeding, dose to the wound may in fact be desirable.
This study used a single beam energy (6 MV), field size (10×10 cm2) and geometry (directly incident beam). Extrapolation to other scenarios relies on previous workReference Morley, Cashell, Sperduti, McQuestion and Chow 5 testing other configurations. The dose implications of the tested products may be higher for lower energy beams, notably for 4 MV or Cobalt 60 sources which are used in some centres. Higher energy beams would result in less superficial dose enhancement. Smaller field sizes, including highly segmented beams such as found in most IMRT and VMAT treatment plans, have lower scatter factors and would therefore result in less dose enhancement. This study did not examine any medical considerations of using these products during RT, such as the effectiveness of these products in preventing, limiting or managing side effects.
CONCLUSIONS
None of the tested products produced a concerning skin dose enhancement under conditions which could reasonably be expected to occur in clinical practice. Very saturated, and absorbent dressings may produce unacceptable skin dose enhancement if they are present for the majority of the treatment course. This conclusion holds for products containing zinc, iodine, silver and those with a sun protection factor. There is no evidence supporting restrictions on the use of these or similar skin products during MV photon beam RT. In this era of new technologies and techniques, medical rationales for designing skin care instructions and wound care management should be pursued to formulate best practice guidelines surrounding usage of topical agents and dressings during RT.
Acknowledgements
Thanks to Tara Rosewall who has provided her writing assistance and support for publication.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of Interest
None.










