Article contents
Tribocorrosion behavior of Ca–P MAO coatings on Ti6Al4V alloy at various applied voltages
Published online by Cambridge University Press: 16 December 2019
Abstract
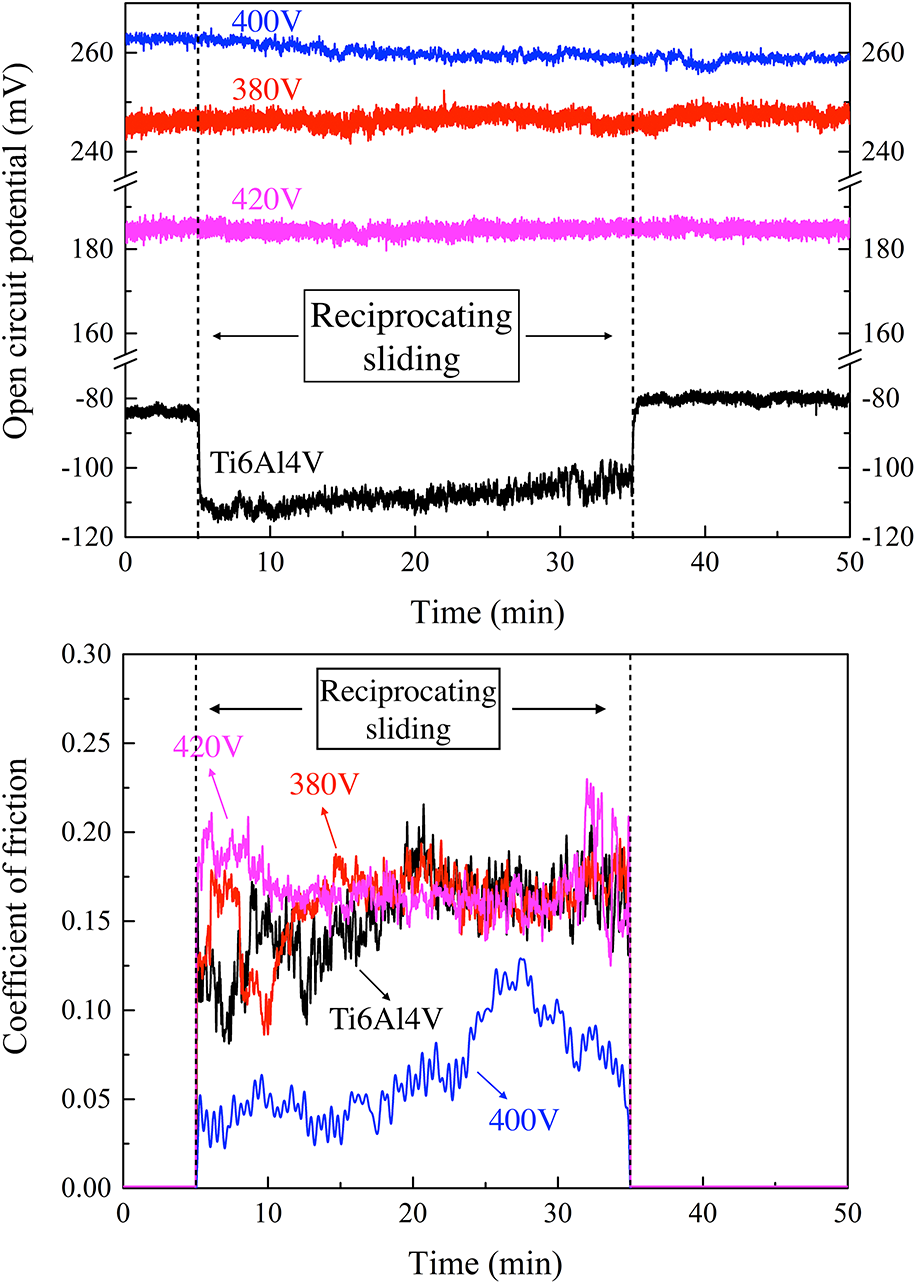
In this study, coatings containing Ca and P elements on Ti6Al4V alloy were fabricated by micro-arc oxidation at different applied voltages. Subsequently, evaluation of the phase structure, morphology, element composition, corrosion mechanism, and tribocorrosion behavior of these coatings was performed. The results showed that the coatings consisted of rutile TiO2 and anatase TiO2. The ratio of rutile/anatase, surface roughness, and hardness increase with the increase of applied voltage. Electrochemical impedance spectroscopy results indicated the corrosion resistance of coatings in simulated body fluid of 400 V > 380 V > 420 V. The open circuit potential of sample 400 V declined during the tribocorrosion test. Sample 420 V possessed the highest wear volume after the tribocorrosion process. The tribocorrosion mechanism of samples 380 and 420 V was mainly confirmed as the wear effect, and the decline of corrosion resistance due to the micro-cracks formed during the abrasive wear of the coating accounts for the tribocorrosion mechanism of sample 400 V.
Keywords
- Type
- Article
- Information
- Journal of Materials Research , Volume 35 , Issue 5: Focus Issue: The Science and Technology of Vapor Phase Processing and Modification of Surfaces , 16 March 2020 , pp. 444 - 453
- Copyright
- Copyright © Materials Research Society 2019
References
- 10
- Cited by


