Article contents
The structure and thermochemistry of K2CO3–MgCO3 glass
Published online by Cambridge University Press: 16 September 2019
Abstract
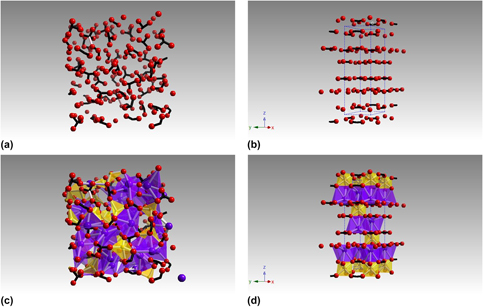
Carbonate glasses can be formed routinely in the system K2CO3–MgCO3. The enthalpy of formation for one such 0.55K2CO3–0.45MgCO3 glass was determined at 298 K to be 115.00 ± 1.21 kJ/mol by drop solution calorimetry in molten sodium molybdate (3Na2O·MoO3) at 975 K. The corresponding heat of formation from oxides at 298 K was −261.12 ± 3.02 kJ/mol. This ternary glass is shown to be slightly metastable with respect to binary crystalline components (K2CO3 and MgCO3) and may be further stabilized by entropy terms arising from cation disorder and carbonate group distortions. This high degree of disorder is confirmed by 13C MAS NMR measurement of the average chemical shift tensor values, which show asymmetry of the carbonate anion to be significantly larger than previously reported values. Molecular dynamics simulations show that the structure of this carbonate glass reflects the strong interaction between the oxygen atoms in distorted carbonate anions and potassium cations.
- Type
- Invited Paper
- Information
- Journal of Materials Research , Volume 34 , Issue 19: Focus Issue: Thermodynamics of Complex Solids , 14 October 2019 , pp. 3377 - 3388
- Copyright
- Copyright © Materials Research Society 2019
Footnotes
Present Address: University of Manchester at Harwell, Diamond Light Source, Harwell Campus, Didcot, Oxfordshire, OX11 0DE, U.K.
This author was an editor of this journal during the review and decision stage. For the JMR policy on review and publication of manuscripts authored by editors, please refer to http://www.mrs.org/editor-manuscripts/.
References
- 6
- Cited by


