Article contents
Solvothermal synthesis of shape-controlled manganese oxide materials and their electrochemical capacitive performances
Published online by Cambridge University Press: 11 September 2013
Abstract
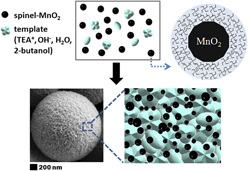
We present a simple and quick procedure for the one-pot synthesis of manganese oxides under a basic solvothermal condition in the presence of cationic surfactants acting as the template in a 2-butanol/water solution. Three-dimensional spinel-type MnO2 microspheres composed of small nanoparticles have been fabricated for the first time using our method. Their corresponding electrochemical performances in the applications of supercapacitor electrodes exhibit a good specific capacitance (SC) value of ∼190 F/g at 0.5 A/g and excellent SC retention and Coulombic efficiency of ∼100% and ∼95% after 1000 charge/discharge cycles at 1 A/g, respectively. This suggests its potential applications in energy storage devices. Further, we demonstrate that this solvothermal technique enables the morphological tuning of manganese oxides in various forms such as schists, rods, fibers, and nanoparticles. This work describes a rapid and low-cost technique to fabricate novel architectures of manganese oxides having the desired crystal phase, which will highly benefit various supercapacitor applications.
- Type
- Articles
- Information
- Journal of Materials Research , Volume 29 , Issue 1: Focus Issue: Synthesis of Nanostructured Functional Oxides , 14 January 2014 , pp. 107 - 114
- Copyright
- Copyright © Materials Research Society 2013
References
REFERENCES
- 4
- Cited by


