Published online by Cambridge University Press: 14 August 2018
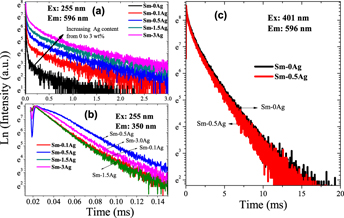
In this paper, Sm3+-doped silicate glasses containing AgNO3 were obtained by the common melting quenching method. Influence of AgNO3 concentration on the absorption and emission characteristics of Sm3+ were systematically investigated. With the increase of AgNO3 content from 0 to 3.0 wt%, the ultraviolet region absorption edge shows a slight blue-shift from 275 to 260 nm. Exciting by 255 nm, the visible emission intensity of Sm3+-doped silicate glass containing 0.5 wt% AgNO3 was about 31 times stronger than that of Sm3+ singly doped silicate glass. Fluorescence decay curves for the visible emission followed double exponential decay. Two fluorescence lifetimes were obtained, one was about 7–20 μs which was comparable with the lifetimes of 350 nm emission which derived from Ag+, another was about 2 ms which was comparable with that of the visible emission from Sm3+ excited by 401 nm. Thus, the significant enhancement visible emission of Sm3+ excited by 255 nm can be ascribed to the energy transfer from Ag+ to Sm3+.
These authors contributed equally to this work.