Article contents
Sensitive detection of mercury (II) ion using wave length-tunable visible-emitting gold nanoclusters based on protein-templated synthesis
Published online by Cambridge University Press: 29 October 2014
Abstract
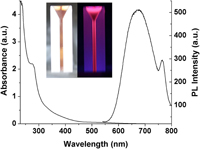
In this study, a simple, facile, green, and versatile strategy was developed for the synthesis of water-soluble and well-dispersed fluorescent gold nanoclusters (Au NCs) with wave length-tunable emissions using protein as capping agent and template. The resultant Au NCs exhibited excellent characteristics such as large Stokes shift, good stability, and high resistance to photobleaching. They showed strong emission at 680 nm, with a quantum yield of 4.5%. Heavy metal ion (Hg2+) could quench the fluorescence of Au NCs efficiently and thus the as-prepared Au NCs were successfully developed as “turn-off” fluorescent probes for the detection of Hg2+ sensitively and selectively. The lowest concentration to quantify mercuric ions could be as low as 1 nM and the fluorescence intensity of the Au NCs was proportional to the concentrations of Hg2+ over the range 8 × 10−8 M to 1 × 10−5 M (R = 0.9982). To validate the practicality of this probe, real water samples spiked with Hg2+ were determined with RSD values less than 3%. And the results demonstrated that the developed probes worked well in environmental samples. The action mechanism between the Au NCs and Hg2+ was explored as well.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2014
References
REFERENCES
- 7
- Cited by


