Article contents
A rapid and scalable method for making mixed metal oxide alloys for enabling accelerated materials discovery
Published online by Cambridge University Press: 28 March 2016
Abstract
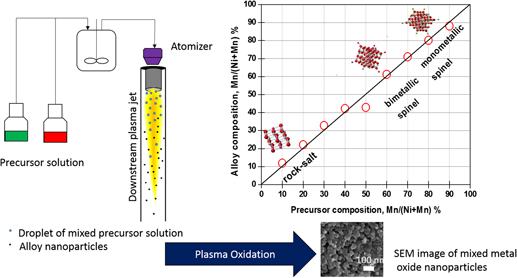
The synthesis technique that can be used to accelerate the discovery of materials for various energy conversion and storage applications is presented. Specifically, this technique allows a rapid and controlled synthesis of mixed metal oxide particles using plasma oxidation of liquid droplets containing mixed metal precursors. The conventional wet chemical methods for synthesis of multimetal oxide solid solutions often require time-consuming high pressure and temperature processes, and so the challenge is to develop rapid and scalable techniques with precise compositional control. The concept is demonstrated by synthesizing binary and ternary transition metal oxide solid solutions with control over entire composition range using metal precursor solution droplets oxidized using atmospheric oxygen plasma. The results show the selective formation of metastable spinel and the rocksalt solid solution phases with compositions over the entire range by tuning the metal precursor composition. The synthesized manganese doped nickel ferrite nanoparticles, NiMn z Fe2−z O4 (0 ≤ z ≤ 1), exhibits considerable electrocatalytic activity toward oxygen evolution reaction, achieving an overpotential of 0.39 V at a benchmarking current density of 10 mA/cm2 for a low manganese content of z = 0.20.
- Type
- Articles
- Information
- Journal of Materials Research , Volume 31 , Issue 11: Focus Issue: Advanced Materials and Structures for Solar Fuels , 14 June 2016 , pp. 1596 - 1607
- Copyright
- Copyright © Materials Research Society 2016
References
REFERENCES
- 13
- Cited by


