Article contents
Preparation, characterization, and luminescent properties of NaGd(WO4)2:Eu3+ nanotubes using carbon nanotubes as templates
Published online by Cambridge University Press: 11 April 2012
Abstract
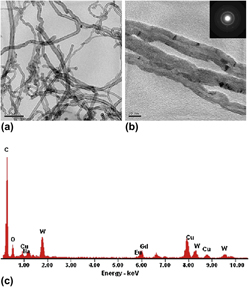
NaGd(WO4)2:Eu3+ nanotubes have been successfully synthesized by the hydrothermal method using carbon nanotubes (CNTs) as removable templates. X-ray diffraction, thermogravimetric and differential thermal analysis, transmission electron microscopy, and photoluminescence were used to characterize the product. It is demonstrated that CNTs are fully coated with an amorphous NaGd(WO4)2:Eu3+ layer, which is about 7 nm thick and almost continuous and uniform. After the NaGd(WO4)2:Eu3+/CNTs composites have been calcined at 500 or 600 °C, NaGd(WO4)2:Eu3+ nanotubes are obtained by removing the CNTs templates, and the outer diameter of that is about 40 nm. The luminescence properties of the NaGd(WO4)2:Eu3+ nanotubes calcined at various temperatures have been investigated. The results indicate that the products exhibit a characteristic red emission peak of Eu3+ ions at 615 nm. The emission intensity decreases with the increasing of annealing temperature, which is probably because a few residual carbons doped in NaGd(WO4)2:Eu3+ nanotubes and many oxygen vacancies could promote the intensity of red emission of Eu3+.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2012
References
REFERENCES
- 3
- Cited by




