Article contents
Photocatalytic activity enhancement of TiO2 porous thin film due to homogeneous surface modification of RuO2
Published online by Cambridge University Press: 16 June 2011
Abstract
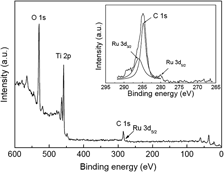
Ruthenium dioxide (RuO2) was uniformly modified on TiO2 porous thin film by impregnation of Ru-contained dye on the film followed by sintering it at 450 °C to burn off organic matters and form ruthenium oxide, which is named as impregnation method. The homogenous modification of metal oxide inside porous thin film can be realized by the impregnation method, and the modification amount of RuO2 can be easily adjusted by the iteration numbers of impregnation and sintering. Appropriate amount of uniformly modified RuO2 was found to obviously enhance photocatalytic performance of TiO2 to degrade eosin Y. The photocatalysis enhancement was attributed to the shallow hole traps on the surface of nanoparticles formed by RuO2, and these traps can retard recombination of hole with electron.
Keywords
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2011
References
REFERENCES
- 9
- Cited by


