Article contents
Oxygen semi-permeation properties of La1−xSrxFeO3−δ perovskite membranes under high oxygen gradient
Published online by Cambridge University Press: 28 August 2020
Abstract
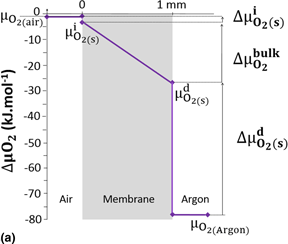
This work is focused on the evaluation of oxygen semi-permeation and electrochemical performances under high oxygen gradient of free cobalt perovskite membrane materials; La1−xSrxFeO3−δ perovskite. For a better understanding of oxygen transport through La1−xSrxFeO3−δ perovskite membranes, the oxygen diffusion, oxygen incorporation, and desorption coefficients were determined under high oxygen gradient in relation to the temperature for La1−xSrxFeO3−δ (with x = 0.1, 0.3, 0.5, and 0.7) by a specific method based on oxygen semi-permeation. The best electrochemical performances were obtained for La0.3Sr0.7FeO3−δ (LSF37) and La0.5Sr0.5FeO3−δ (LSF55) perovskite membranes with oxygen fluxes of 1.7 × 10−3 and 1.2 × 10−3 mol/m2 s at 900 °C, respectively. The oxygen incorporation and desorption coefficients of LSF55 were two times lower than those of LSF37 and similar to those of La0.5Sr0.5Fe0.7Ga0.3O3−δ. The values of these coefficients are discussed and compared with the data reported in the literature by isotopic exchange for the similar material compositions.
- Type
- Article
- Information
- Copyright
- Copyright © Materials Research Society 2020
References
- 7
- Cited by



