Article contents
Oriented growth of single NaCl (100) crystal induced by Langmuir–Blodgett film
Published online by Cambridge University Press: 24 January 2011
Abstract
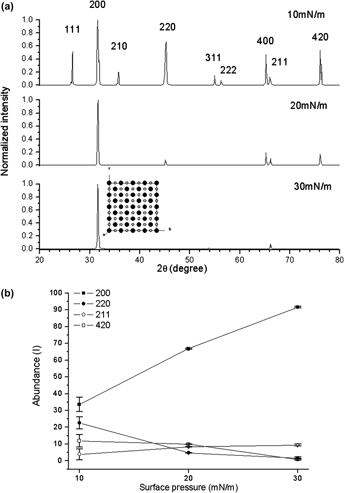
Molecular films have been extensively used for crystal growth. The Langmuir–Blodgett (LB) technique allows the oriented molecules film to be produced in a controllable manner. By controlling the LB film surface pressure and tuning quenching temperature of the supersaturated solution, single NaCl (100) crystal planes are successfully produced under the surface pressure of 30 mN/m with quenching temperature 5 °C of the NaCl supersaturated solution. The mechanism of single NaCl (100) crystal growth can be further explained based on the best matching between NaCl (100) crystal plane distance and the lattice parameter of LB film among all other crystal planes.
- Type
- Reviews
- Information
- Journal of Materials Research , Volume 26 , Issue 2: Focus Issue: Self-Assembly and Directed Assembly of Advanced Materials , 28 January 2011 , pp. 230 - 235
- Copyright
- Copyright © Materials Research Society 2011
References
REFERENCES
- 2
- Cited by




